- Record: found
- Abstract: found
- Article: found
In Vitro Intraductal MRI and T2 Mapping of Cholangiocarcinoma Using Catheter Coils
Read this article at
Abstract
Aim
Diagnostic imaging of early-stage cholangiocarcinoma is challenging. A previous in vitro study of fixed-tissue liver resection specimens investigated T2 mapping as a method of exploiting the locally increased signal-to-noise ratio (SNR) of duodenoscope coils for improved quantitative magnetic resonance imaging (MRI), despite their non-uniform sensitivity. This work applies similar methods to unfixed liver specimens using catheter-based receivers.
Methods
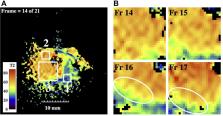
Ex vivo intraductal MRI and T2 mapping were carried out at 3T on unfixed resection specimens obtained from cholangiocarcinoma patients immediately after surgery using a catheter coil based on a thin-film magneto-inductive waveguide, inserted directly into an intrahepatic duct.
Results
Polypoid intraductal cholangiocarcinoma was imaged using fast spin-echo sequences. High-resolution T2 maps were extracted by fitting of data obtained at different echo times to mono-exponential models, and disease-induced changes were correlated with histopathology. An increase in T2 was found compared with fixed specimens and differences in T2 allowed the resolution of tumour tissue and malignant features such as polypoid morphology.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Cholangiocarcinoma.
- Record: found
- Abstract: found
- Article: not found
The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer.
- Record: found
- Abstract: found
- Article: not found