- Record: found
- Abstract: found
- Article: found
Initiation of ART during Early Acute HIV Infection Preserves Mucosal Th17 Function and Reverses HIV-Related Immune Activation
Read this article at
Abstract
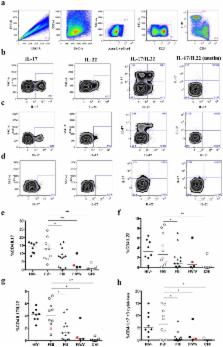
Mucosal Th17 cells play an important role in maintaining gut epithelium integrity and thus prevent microbial translocation. Chronic HIV infection is characterized by mucosal Th17 cell depletion, microbial translocation and subsequent immune-activation, which remain elevated despite antiretroviral therapy (ART) correlating with increased mortality. However, when Th17 depletion occurs following HIV infection is unknown. We analyzed mucosal Th17 cells in 42 acute HIV infection (AHI) subjects (Fiebig (F) stage I-V) with a median duration of infection of 16 days and the short-term impact of early initiation of ART. Th17 cells were defined as IL-17+ CD4+ T cells and their function was assessed by the co-expression of IL-22, IL-2 and IFNγ. While intact during FI/II, depletion of mucosal Th17 cell numbers and function was observed during FIII correlating with local and systemic markers of immune-activation. ART initiated at FI/II prevented loss of Th17 cell numbers and function, while initiation at FIII restored Th17 cell numbers but not their polyfunctionality. Furthermore, early initiation of ART in FI/II fully reversed the initially observed mucosal and systemic immune-activation. In contrast, patients treated later during AHI maintained elevated mucosal and systemic CD8+ T-cell activation post initiation of ART. These data support a loss of Th17 cells at early stages of acute HIV infection, and highlight that studies of ART initiation during early AHI should be further explored to assess the underlying mechanism of mucosal Th17 function preservation.
Author Summary
Persistent systemic immune activation is a hallmark of chronic HIV infection and an independent predictor of disease progression. The underlying mechanism is not yet completely understood but thought to be associated with the loss of Th17 cells leading to the disruption of the mucosal barrier and subsequent microbial translocation. However, it remains unclear when these events take place in HIV infection, as the only data available to date are from SIV models. We evaluated the kinetics of Th17 depletion, microbial translocation and subsequent immune activation in early acute HIV infection and the effect of early initiated ART on these events. We discovered that a collapse of Th17 cell number and function, accompanied by local and systemic immune activation, occurs already during acute HIV infection. However, early initiation of ART preserved Th17 number and function and fully reversed any initial HIV-related immune activation. These findings argue for the importance of early events during HIV infection setting the stage for chronic immune activation and for early and aggressive treatment during acute HIV infection.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
The immune response during acute HIV-1 infection: clues for vaccine development
- Record: found
- Abstract: found
- Article: not found
Induction and effector functions of T(H)17 cells.
- Record: found
- Abstract: found
- Article: not found