- Record: found
- Abstract: found
- Article: found
EGFR and K-Ras mutations in women with lung adenocarcinoma: implications for treatment strategy definition
Read this article at
Abstract
Background
We aimed at investigating the outcomes of female patients with stage IIIB-IV adenocarcinoma of the lung according to EGFR and K-Ras mutational status.
Methods
One hundred and three consecutive female patients genotyped at a single Italian Institution were analyzed. Patients were planned to receive first-line platinum-based chemotherapy (CT) and a salvage treatment with anti-EGFR tyrosine-kinase inhibitors (TKIs) was proposed irrespective of tumor mutational status. EGFR (exons 18–21) and K-Ras (exon 2, codons 12–13) mutations were evaluated by real-time PCR and pyrosequencing. The association of mutational status with clinical variables and treatment benefit was investigated by chi-square test and log-rank test.
Results
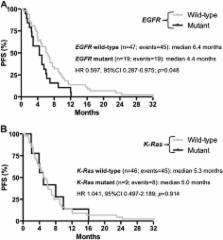
EGFR and K-Ras mutations were found in 31 (30%) and 13 (15%) cases, respectively. Sixty-six patients received platinum CT: no correlation was observed between EGFR or K-Ras mutational status and response rate (RR) (p > 0.05). However, patients treated with first-line CT harboring EGFR activating mutations experienced a significantly reduced progression-free survival (PFS) in comparison with wild-type ones (4.4 vs. 6.4 months, respectively; HR 0.597, 95% CI 0.287-0.975; p = 0.048). Thirty-nine patients received salvage treatment with erlotinib: EGFR activating mutations were significantly correlated with RR (60% vs. 12.5%; p = 0.004) and PFS (11.4 vs. 4.5 months; HR 0.491, 95% CI 0.216-0.936; p = 0.044). Responses to erlotinib were not reported among women with K-Ras mutant tumors, while 50% of those with wild-type K-Ras achieved an objective remission (p = 0.296). Median PFS (3.5 vs. 8.8 months; HR 0.284, 95% CI 0.015-0.510; p = 0.010) and OS (3.9 vs. 19.8 months; HR 0.158, 95% CI 0.001-0.075; p < 0.001) were significantly shorter among K-Ras mutant patients treated with TKI.
Conclusions
In our population of Caucasian women with advanced lung adenocarcinoma we observed that the presence of EGFR activating mutations correlates with a significant reduction in the benefit from first-line platinum-based CT, emphasizing the importance of an upfront use of anti-EGFR TKIs in this patient subset. K-Ras mutations seem to correlate with a detrimental effect from anti-EGFR TKI, but this finding deserves further investigation.
Related collections
Most cited references14
- Record: found
- Abstract: found
- Article: not found
Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer.
- Record: found
- Abstract: found
- Article: not found
KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies.
- Record: found
- Abstract: found
- Article: not found
