- Record: found
- Abstract: found
- Article: found
Treatment decisions, clinical outcomes, and pharmacoeconomics in the treatment of patients with EGFR mutated stage III/IV NSCLC in Germany: an observational study
Read this article at
Abstract
Background
We evaluated treatment decisions and outcomes in a cohort of predominately Caucasian patients with EGFR mutation-positive ( EGFR Mut+) non-small-cell lung cancer (NSCLC).
Methods
REASON (NCT00997230) was a non-interventional study in German patients with stage IIIB/IV NSCLC. Secondary endpoints for EGFR Mut + NSCLC included progression-free survival (PFS), overall survival (OS), adverse event (AE) management, and pharmacoeconomic outcomes.
Results
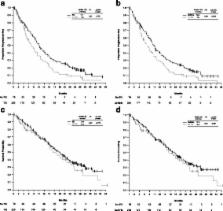
Among 334 patients with EGFR Mut + NSCLC, tyrosine kinase inhibitors (TKIs) were the most common first-line therapy (56.6%, 53.0% gefitinib). Among patients who received TKIs/gefitinib before first disease progression, PFS was longer compared with those who did not receive a TKI (median 10.1/10.0 vs. 7.0 months; HR 0.67/0.69; log-rank p = 0.012/ p = 0.022). OS was longer for those patients who ever received a TKI/gefitinib during their complete therapy course compared with those who never received a TKI (median 18.4/18.1 vs. 13.6 months; HR 0.53/0.55; p = 0.003/ p = 0.005). Total mean first-line treatment healthcare costs per person were higher for those receiving TKIs (€46,443) compared with those who received chemotherapy (€27,182). Mean outpatient and inpatient costs were highest with chemotherapy. Rash, diarrhea, and dry skin were the most commonly reported AEs for patients receiving gefitinib.
Conclusions
In REASON, TKI therapy was the most common first- and second-line treatment for EGFR Mut + NSCLC, associated with increased drug costs compared with chemotherapy. Patients who received gefitinib or a TKI ever during their complete therapy course had prolonged PFS and OS compared with patients who did not receive a TKI.
Trial registration
The trial was registered on October, 2009 with ClinicalTrials.gov: https://clinicaltrials.gov/ct2/show/NCT00997230?term=NCT00997230&rank=1
Related collections
Most cited references10
- Record: found
- Abstract: found
- Article: not found
Genotyping and genomic profiling of non-small-cell lung cancer: implications for current and future therapies.
- Record: found
- Abstract: found
- Article: not found
A genomics-based classification of human lung tumors.
- Record: found
- Abstract: found
- Article: not found