- Record: found
- Abstract: found
- Article: not found
Cancer-cell stiffening via cholesterol depletion enhances adoptive T-cell immunotherapy
Read this article at
Abstract
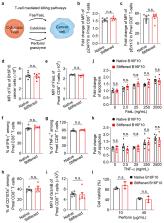
Malignancy and tumour progression are associated with cancer-cell softening. Yet how the biomechanics of cancer cells affects T-cell mediated cytotoxicity and thus the outcomes of adoptive T-cell immunotherapies is unknown. Here, we show that T-cell-mediated cancer-cell killing is hampered for cortically soft cancer cells, whose plasma membrane is enriched with cholesterol, and that cancer-cell stiffening via cholesterol depletion augments T-cell cytotoxicity and enhances the efficacy of adoptive T-cell therapy against solid tumours in mice. We also show that the enhanced cytotoxicity against stiffened cancer cells is mediated by augmented T-cell forces arising from an increased accumulation of filamentous actin at the immunological synapse, and that cancer-cell stiffening has a negligible influence on T-cell-receptor signalling, on the production of cytolytic proteins such as granzyme B, on the secretion of interferon gamma and tumour necrosis factor alpha, and on Fas-receptor–Fas-ligand interactions. Our findings reveal a mechanical immune checkpoint that could be targeted therapeutically to improve the effectiveness of cancer immunotherapies.
Cancer cells enriched with cholesterol in their plasma membrane impair T-cell mediated cytotoxicity, which can be augmented by stiffening the cancer cells via cholesterol depletion, as shown in mouse models of adoptive T-cell therapy.
Related collections
Most cited references63
- Record: found
- Abstract: found
- Article: found
Hallmarks of Cancer: The Next Generation
- Record: found
- Abstract: found
- Article: not found
Cancer immunotherapy using checkpoint blockade
- Record: found
- Abstract: found
- Article: not found
