- Record: found
- Abstract: found
- Article: found
Local accumbens in vivo imaging during deep brain stimulation reveals a strategy-dependent amelioration of hedonic feeding
Read this article at
Significance
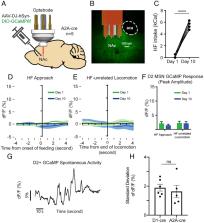
Impulsive overeating is a common, disabling feature of eating disorders. Calcium imaging using fiber photometry has emerged as an in vivo methodology to measure neuronal population activity immune to electrical stimulation artifact from deep brain stimulation (DBS). Thus, when used simultaneously, calcium imaging can elucidate poorly understood DBS mechanisms. We show that nucleus accumbens D1 medial spiny calcium signaling increases in preparation of hedonic feeding of high-fat food. Further, responsive, over continuous, DBS strategies effectively disrupt this activity leading to decreased consumption. Implementation of this methodology to better understand mechanisms of these and other forms of neuromodulation for various indications may help advance the field to identify novel therapeutic targets with applications extending beyond obesity.
Abstract
Impulsive overeating is a common, disabling feature of eating disorders. Both continuous deep brain stimulation (DBS) and responsive DBS, which limits current delivery to pathological brain states, have emerged as potential therapies. We used in vivo fiber photometry in wild-type, Drd1-cre, and A2a-cre mice to 1) assay subtype-specific medium spiny neuron (MSN) activity of the nucleus accumbens (NAc) during hedonic feeding of high-fat food, and 2) examine DBS strategy-specific effects on NAc activity. D1, but not D2, NAc GCaMP activity increased immediately prior to high-fat food approach. Responsive DBS triggered a GCaMP surge throughout the stimulation period and durably reduced high-fat intake. However, with continuous DBS, this surge decayed, and high-fat intake reemerged. Our results argue for a stimulation strategy-dependent modulation of D1 MSNs with a more sustained decrease in consumption with responsive DBS. This study illustrates the important role in vivo imaging can play in understanding effects of such novel therapies.
Related collections
Most cited references46
- Record: found
- Abstract: found
- Article: not found
The prevalence and correlates of eating disorders in the National Comorbidity Survey Replication.
- Record: found
- Abstract: found
- Article: not found
Natural neural projection dynamics underlying social behavior.
- Record: found
- Abstract: found
- Article: not found