- Record: found
- Abstract: found
- Article: not found
Proteomic Analysis of Altered Extracellular Matrix Turnover in Bleomycin-induced Pulmonary Fibrosis
Read this article at
Abstract
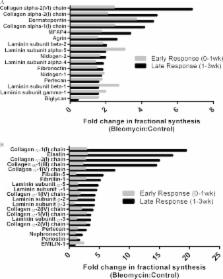
Fibrotic disease is characterized by the pathological accumulation of extracellular matrix (ECM) proteins. Surprisingly, very little is known about the synthesis and degradation rates of the many proteins and proteoglycans that constitute healthy or pathological extracellular matrix. A comprehensive understanding of altered ECM protein synthesis and degradation during the onset and progression of fibrotic disease would be immensely valuable. We have developed a dynamic proteomics platform that quantifies the fractional synthesis rates of large numbers of proteins via stable isotope labeling and LC/MS-based mass isotopomer analysis. Here, we present the first broad analysis of ECM protein kinetics during the onset of experimental pulmonary fibrosis. Mice were labeled with heavy water for up to 21 days following the induction of lung fibrosis with bleomycin. Lung tissue was subjected to sequential protein extraction to fractionate cellular, guanidine-soluble ECM proteins and residual insoluble ECM proteins. Fractional synthesis rates were calculated for 34 ECM proteins or protein subunits, including collagens, proteoglycans, and microfibrillar proteins. Overall, fractional synthesis rates of guanidine-soluble ECM proteins were faster than those of insoluble ECM proteins, suggesting that the insoluble fraction reflected older, more mature matrix components. This was confirmed through the quantitation of pyridinoline cross-links in each protein fraction. In fibrotic lung tissue, there was a significant increase in the fractional synthesis of unique sets of matrix proteins during early (pre-1 week) and late (post-1 week) fibrotic response. Furthermore, we isolated fast turnover subpopulations of several ECM proteins ( e.g. type I collagen) based on guanidine solubility, allowing for accelerated detection of increased synthesis of typically slow-turnover protein populations. This establishes the presence of multiple kinetic pools of pulmonary collagen in vivo with altered turnover rates during evolving fibrosis. These data demonstrate the utility of dynamic proteomics in analyzing changes in ECM protein turnover associated with the onset and progression of fibrotic disease.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
An overview of tissue and whole organ decellularization processes.
- Record: found
- Abstract: found
- Article: not found
