- Record: found
- Abstract: found
- Article: not found
Enhanced Electrochemical Stability of Molten Li Salt Hydrate Electrolytes by the Addition of Divalent Cations

Read this article at
Abstract
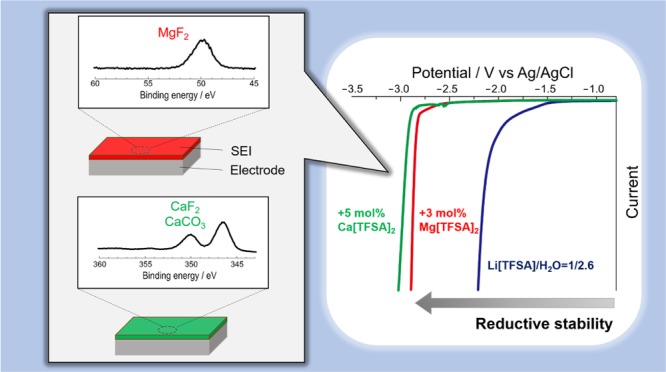
Water can be an attractive solvent for Li-ion battery electrolytes owing to numerous advantages such as high polarity, nonflammability, environmental benignity, and abundance, provided that its narrow electrochemical potential window can be enhanced to a similar level to that of typical nonaqueous electrolytes. In recent years, significant improvements in the electrochemical stability of aqueous electrolytes have been achieved with molten salt hydrate electrolytes containing extremely high concentrations of Li salt. In this study, we investigated the effect of divalent salt additives (magnesium and calcium bis(trifluoromethanesulfonyl)amides) in a molten salt hydrate electrolyte (21 mol kg –1 lithium bis(trifluoromethanesulfonyl)amide) on the electrochemical stability and aqueous lithium secondary battery performance. We found that the electrochemical stability was further enhanced by the addition of the divalent salt. In particular, the reductive stability was increased by more than 1 V on the Al electrode in the presence of either of the divalent cations. Surface characterization with X-ray photoelectron spectroscopy suggests that a passivation layer formed on the Al electrode consists of inorganic salts (most notably fluorides) of the divalent cations and the less-soluble solid electrolyte interphase mitigated the reductive decomposition of water effectively. The enhanced electrochemical stability in the presence of the divalent salts resulted in a more-stable charge–discharge cycling of LiCoO 2 and Li 4Ti 5O 12 electrodes.
Related collections
Most cited references56
- Record: found
- Abstract: not found
- Article: not found
Electrolytes and interphases in Li-ion batteries and beyond.
- Record: found
- Abstract: found
- Article: not found
"Water-in-salt" electrolyte enables high-voltage aqueous lithium-ion chemistries.
- Record: found
- Abstract: not found
- Article: not found
