- Record: found
- Abstract: found
- Article: found
Neutrophil elastase inhibition effectively rescued angiopoietin-1 decrease and inhibits glial scar after spinal cord injury
Read this article at
Abstract
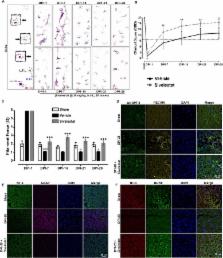
After spinal cord injury (SCI), neutrophil elastase (NE) released at injury site disrupts vascular endothelium integrity and stabilization. Angiopoietins (ANGPTs) are vascular growth factors that play an important role in vascular stabilization. We hypothesized that neutrophil elastase is one of the key determinants of vascular endothelium disruption/destabilization and affects angiopoietins expression after spinal cord injury. To test this, tubule formation and angiopoietins expression were assessed in endothelial cells exposed to different concentrations of recombinant neutropil elastase. Then, the expression of angiopoietin-1, angiopoietin-2, and neutrophil elastase was determined at 3 h and at 1, 3, 5, 7, 14, 21, and 28 days in a clinically relevant model of moderate compression (35 g for 5 min at T10) spinal cord injury. A dichotomy between the levels of angiopoietin-1 and angiopoietin-2 was observed; thus, we utilized a specific neutrophil elastase inhibitor (sivelestat sodium; 30 mg/kg, i.p., b.i.d.) after spinal cord injury. The expression levels of neutropil elastase and angiopoietin-2 increased, and that of angiopoietin-1 decreased after spinal cord injury in rats. The sivelestat regimen, optimized via a pharmacokinetics study, had potent effects on vascular stabilization by upregulating angiopoietin-1 via the AKT pathway and preventing tight junction protein degradation. Moreover, sivelestat attenuated the levels of inflammatory cytokines and chemokines after spinal cord injury and hence subsequently alleviated secondary damage observed as a reduction in glial scar formation and the promotion of blood vessel formation and stabilization. As a result, hindlimb locomotor function significantly recovered in the sivelestat-treated animals as determined by the Basso, Beattie, and Bresnahan scale and footprint analyses. Furthermore, sivelestat treatment attenuated neuropathic pain as assessed by responses to von Frey filaments after spinal cord injury. Thus, our result suggests that inhibiting neutropil elastase by administration of sivelestat is a promising therapeutic strategy to inhibit glial scar and promote functional recovery by upregulating angiopoietin-1 after spinal cord injury.
Related collections
Most cited references76
- Record: found
- Abstract: found
- Article: not found
Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis.
- Record: found
- Abstract: found
- Article: not found