- Record: found
- Abstract: found
- Article: found
Inhibition of Long Noncoding RNA SNHG15 Ameliorates Hypoxia/Ischemia-Induced Neuronal Damage by Regulating miR-302a-3p/STAT1/NF-κB Axis
Read this article at
Abstract
Purpose
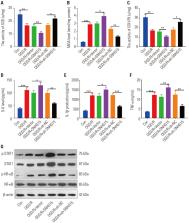
Ischemic brain injury results in high mortality and serious neurologic morbidity. Here, we explored the role of SNHG15 in modulating neuronal damage and microglial inflammation after ischemia stroke.
Materials and Methods
The hypoxia/ischemia models were induced by middle cerebral artery occlusion in mice and oxygen-glucose deprivation/reoxygenation (OGD/R) in vitro. Quantitative real-time PCR (qRT-PCR) and Western blot were conducted to determine the levels of SNHG15, miR-302a-3p, and STAT1/NF-κB. Moreover, gain- or loss-of functional assays of SNHG15 and miR-302a-3p were conducted. MTT assay was used to evaluate the viability of HT22 cells, and the apoptotic level was determined by flow cytometry. Furthermore, enzyme-linked immunosorbent assay was performed to detect oxidative stress and inflammatory mediators in the ischemia cortex and OGD/R-treated BV2 microglia.
Results
The SNHG15 and STAT1/NF-κB pathways were both distinctly up-regulated, while miR-302a-3p was notably down-regulated in the ischemia cortex. Additionally, overexpressing SNHG15 dramatically enhanced OGD/R-mediated neuronal apoptosis as well as the expression of oxidative stress and inflammation factors from microglia. In contrast, knocking down SNHG15 or overexpressing miR-302a-3p relieved OGD/R-mediated neuronal apoptosis and microglial activation. Moreover, the rescue experiment testified that overexpressing miR-302a-3p also attenuated SNHG15 up-regulation-induced effects. In terms of the mechanisms, SNHG15 sponged miR-302a-3p and activated STAT1/NF-κB as a competitive endogenous RNA, while miR-302a-3p targeted STAT1 and negatively regulated the STAT1/NF-κB pathway.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here.
- Record: found
- Abstract: found
- Article: not found
Brain-immune interactions in perinatal hypoxic-ischemic brain injury
- Record: found
- Abstract: found
- Article: not found