- Record: found
- Abstract: found
- Article: found
Regulation of hematogenous tumor metastasis by acid sphingomyelinase
Read this article at
Abstract
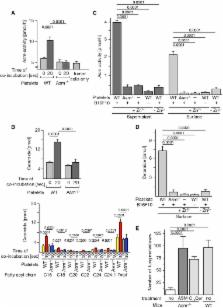
Metastatic dissemination of cancer cells is the ultimate hallmark of malignancy and accounts for approximately 90% of human cancer deaths. We investigated the role of acid sphingomyelinase (Asm) in the hematogenous metastasis of melanoma cells. Intravenous injection of B16F10 melanoma cells into wild-type mice resulted in multiple lung metastases, while Asm-deficient mice ( Smpd1 −/− mice) were protected from pulmonary tumor spread. Transplanting wild-type platelets into Asm-deficient mice reinstated tumor metastasis. Likewise, Asm-deficient mice were protected from hematogenous MT/ ret melanoma metastasis to the spleen in a mouse model of spontaneous tumor metastasis. Human and mouse melanoma cells triggered activation and release of platelet secretory Asm, in turn leading to ceramide formation, clustering, and activation of α5β1 integrins on melanoma cells finally leading to adhesion of the tumor cells. Clustering of integrins by applying purified Asm or C 16 ceramide to B16F10 melanoma cells before intravenous injection restored trapping of tumor cells in the lung in Asm-deficient mice. This effect was revertable by arginine-glycine-aspartic acid peptides, which are known inhibitors of integrins, and by antibodies neutralizing β1 integrins. These findings indicate that melanoma cells employ platelet-derived Asm for adhesion and metastasis.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found
Metastasis: a question of life or death.
- Record: found
- Abstract: found
- Article: not found
Integrin signaling.
- Record: found
- Abstract: found
- Article: not found