- Record: found
- Abstract: found
- Article: found
The association of tadalafil exposure with lower rates of major adverse cardiovascular events and mortality in a general population of men with erectile dysfunction

Read this article at
Abstract
Background
Tadalafil is a long‐acting phosphodiesterase‐5 inhibitor (PDE‐5i) indicated for erectile dysfunction (ED).
Hypothesis
Our hypothesis was that tadalafil will reduce the risk of major adverse cardiovascular events (MACE: composite of cardiovascular death, myocardial infarction, coronary revascularization, unstable angina, heart failure, stroke) and all‐cause death in men with ED.
Methods
A retrospective observational cohort study was conducted in a large US commercial insurance claims database in men with a diagnosis of ED without prior MACE within 1 year. The exposed group ( n = 8156) had ≥1 claim for tadalafil; the unexposed group ( n = 21 012) had no claims for any PDE‐5i.
Results
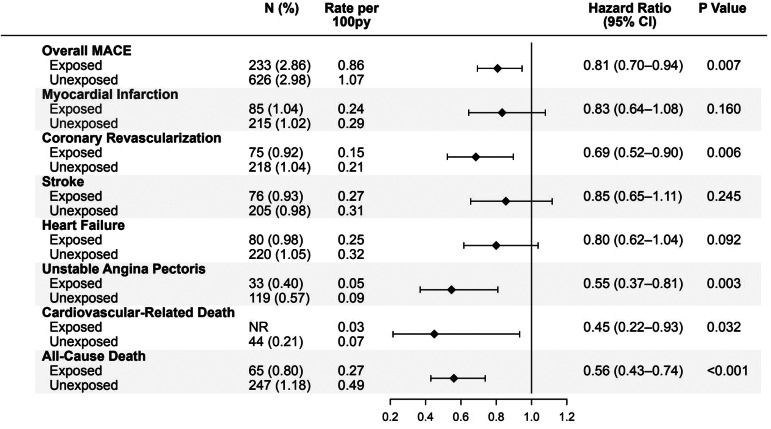
Primary outcome was MACE; secondary outcome was all‐cause death. Groups were matched for cardiovascular risk factors, including preventive therapy. Over a mean follow‐up of 37 months for the exposed group and 29 months for the unexposed group, adjusted rates of MACE were 19% lower in men exposed to tadalafil versus those unexposed to any PDE‐5i (hazard ratio [HR] = 0.81; 95% confidence intervals [CI] = 0.70−0.94; p = .007). Tadalafil exposure was associated with lower adjusted rates of coronary revascularization (HR = 0.69; 95% CI = 0.52−0.90; p = .006); unstable angina (HR = 0.55; 95% CI = 0.37−0.81; p = .003); and cardiovascular‐related mortality (HR = 0.45; CI = 0.22−0.93; p = .032). Overall mortality rate was 44% lower in men exposed to tadalafil (HR = 0.56; CI = 0.43−0.74; p < .001). Men in the highest quartile of tadalafil exposure had the lowest rates of MACE (HR: 0.40; 95% CI: 0.28−0.58; p < .001) compared to lowest exposure quartile.
Abstract
Forest plot demonstrating that men with erectile dysfunction (ED) who were exposed to tadalafil ( N = 8156), had significantly lower adjusted rates of major adverse cardiovascular events (hazard ratio [HR]: 0.81), coronary revascularization (HR: 0.69), unstable angina (HR: 0.55), cardiovascular related deaths (HR: 0.45), and all cause death (HR: 0.56), compared to closely matched men with ED not exposed to tadalafil or other PDE‐5 inhibitors ( N = 21 012). Py, patient‐years.
Related collections
Most cited references8

- Record: found
- Abstract: found
- Article: found
Association between treatment for erectile dysfunction and death or cardiovascular outcomes after myocardial infarction

- Record: found
- Abstract: found
- Article: found
Phosphodiesterase type-5 inhibitor use in type 2 diabetes is associated with a reduction in all-cause mortality

- Record: found
- Abstract: found
- Article: found