- Record: found
- Abstract: found
- Article: not found
Ecdysteroid 7,9(11)-dien-6-ones as potential photoaffinity labels for ecdysteroid binding proteins
Read this article at
Abstract
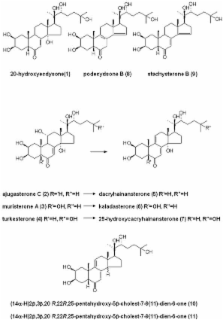
Three ecdysteroid 7,9(11)-dien-7-ones (dacryhainansterone, 25-hydroxydacryhainansterone and kaladasterone) were prepared by dehydration of the corresponding 11a-hydroxy ecdysteroids (ajugasterone C, turkesterone and muristerone A, respectively). The biological activities of the dienones in the Drosophila melanogaster B II cell bioassay, which reflect the affinity for the ecdysteroid receptor complex, showed that the dienones retain high biological activity. Irradiation at 350 nm of the ecdysteroid dienones (100 nM) with bacterially-expressed dipteran and lepidopteran ecdysteroid receptor proteins (DmEcR/DmUSP or CfEcR/CfUSP), followed by loading with [ 3H]ponasterone A revealed that irradiation of dacryhainansterone or kaladasterone resulted in blocking of >70% of the specific binding sites. Thus, ecdysteroid dienones show considerable potential as photoaffinity analogues for ecdysteroid binding proteins.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
Functional ecdysone receptor is the product of EcR and Ultraspiracle genes.
- Record: found
- Abstract: found
- Article: not found
New insecticides with ecdysteroidal and juvenile hormone activity.
- Record: found
- Abstract: found
- Article: not found