- Record: found
- Abstract: found
- Article: found
Randomised clinical trial: a dose‐ranging study of vonoprazan, a novel potassium‐competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis
Read this article at
Summary
Background
The potassium‐competitive acid blocker vonoprazan ( VPZ) has potent acid‐inhibitory effects and may offer clinical advantages over conventional therapy for acid‐related disorders.
Methods
In this multicentre, randomised, double‐blind, parallel‐group, dose‐ranging study, patients ≥20 years with endoscopically confirmed EO [Los Angeles ( LA) grades A−D] received VPZ 5, 10, 20 or 40 mg, or lansoprazole ( LPZ) 30 mg once daily for 8 weeks. The primary endpoint was the proportion of healed EO subjects as shown by endoscopy at week 4.
Results
A total of 732 subjects received VPZ or LPZ. The proportion of healed EO subjects at week 4 was 92.3%, 92.5%, 94.4%, 97.0% and 93.2%, respectively, with VPZ 5, 10, 20 and 40 mg and LPZ 30 mg. All VPZ doses were non‐inferior to LPZ when adjusted for baseline LA grades A/B and C/D. Among those with LA grades C/D, the proportions of healed EO subjects were 87.3%, 86.4%, 100%, 96.0% and 87.0%, respectively, with VPZ 5, 10, 20 and 40 mg and LPZ 30 mg. The incidence of adverse events was similar across the groups.
Conclusions
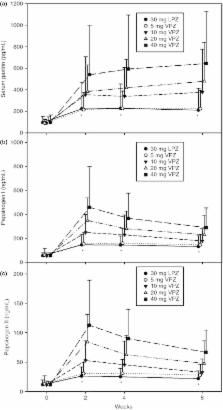
Vonoprazan was effective and non‐inferior to LPZ in healing EO. VPZ 20 mg or higher was highly efficacious for severe EO ( LA grades C/D). VPZ was associated with no safety concern during this 8‐week study, while there was a dose‐dependent increase in serum gastrin. Once‐daily VPZ 20 mg is the recommended clinical dose for treating EO.
Related collections
Most cited references29
- Record: found
- Abstract: found
- Article: not found
Epidemiology and clinical characteristics of GERD in the Japanese population.

- Record: found
- Abstract: found
- Article: not found