- Record: found
- Abstract: found
- Article: found
Synthesis of dienes from pyrrolidines using skeletal modification
Read this article at
Abstract
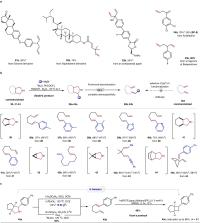
Saturated N-heterocyclic pyrrolidines are common in natural products, medicinal compounds and agrochemicals. However, reconstruction of their skeletal structures creating new chemical space is a challenging task, and limited methods exist for this purpose. In this study, we report a skeletal modification strategy for conversion of polar cyclic pyrrolidines into nonpolar linear dienes through a N-atom removal and deconstruction process. This involves N-sulfonylazidonation followed by rearrangement of the resulting sulfamoyl azide intermediates. This can be an energetically unfavorable process, which involves the formation of active C–C π bonds, the consumption of inert C–N and C–C σ bonds and the destruction of stable five-membered rings, but we have used it here to produce versatile conjugated and nonconjugated dienes with links of varying lengths. We also studied the application of this method in late-stage skeletal modification of bioactive compounds, formal traceless C(sp 2)–H functionalization and formal N-atom deletion.
Abstract
Saturated N-heterocyclic pyrrolidines are common in natural products and medicinal compounds, but reconstruction of their skeletal structures to access new chemical space is a challenging. Here, the authors report a skeletal modification strategy for conversion of polar cyclic pyrrolidines into nonpolar linear dienes through a N-atom removal and deconstruction process.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals.
- Record: found
- Abstract: found
- Article: not found
Efficient visible light photocatalysis of [2+2] enone cycloadditions.
- Record: found
- Abstract: not found
- Article: not found