- Record: found
- Abstract: found
- Article: not found
The Protective Role of Pregnane X Receptor in Lipopolysaccharide/D-galactosamine-induced Acute Liver Injury
Read this article at
Abstract
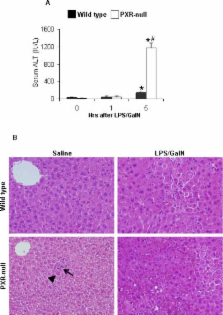
The pregnane x receptor (PXR) is a nuclear receptor transcription factor regulating drug-metabolizing enzymes and transporters that facilitate xenobiotic and endobiotic detoxification. Recent studies demonstrate that PXR is important in abrogating intestinal tissue damage. This study examines the role of PXR in lipopolysaccharide (LPS)/D-galactosamine (GalN)-induced acute liver injury using wild type and PXR-null mice. LPS/GalN-treated PXR-null mice had greater increases of alanine aminotransferase (ALT), hepatocyte apoptosis, necrosis, and hemorrhagic liver injury than wild type mice. LPS/GalN-mediated phosphorylation of JNK1/2 and ERK1/2 was differentially regulated in wild type and PXR-null mice. Importantly, LPS/GalN-induced hepatic Stat3 survival signaling was impaired and early activation of Jak2 was delayed in PXR-null mice. Expression levels of pro-survival proteins Bcl-xL and heme oxygenase-1 (HO-1), which are downstream of Stat3, were substantially lower in PXR-null than wild type mouse livers after LPS/GalN treatment. Autophagy is also involved in LPS/GalN-induced liver injury. Lack of PXR resulted in a significant reduction of LC3B-I, -II as well as Beclin-1 protein levels after LPS/GalN treatment. Taken together, PXR is a critical hepatoprotective factor. Increases of LPS/GalN-induced hepatocyte apoptosis and liver injury in PXR-null mice are due to deregulated MAP kinase activation as well as delayed Jak2/Stat3 activation, which lead to a compromise in defense mechanisms that involve Bcl-xL-, HO-1, and autophagy-mediated pathways.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway.
- Record: found
- Abstract: found
- Article: not found
Stat3 as an oncogene.
- Record: found
- Abstract: found
- Article: not found
