- Record: found
- Abstract: not found
- Article: not found
The level of 24-Hydroxycholesteryl Esters is an Early Marker of Alzheimer’s Disease
Author(s):
Luisa Benussi
1 ,
Roberta Ghidoni
1 ,
Fabrizio Dal Piaz
2 ,
Giuliano Binetti
1
,
3 ,
Giuseppe Di Iorio
4 ,
Paolo Abrescia
5
Publication date (Print):
January 24 2017
Journal:
Journal of Alzheimer's Disease
Publisher:
IOS Press
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references28
- Record: found
- Abstract: found
- Article: not found
Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force.
F Deisenhammer, A Bartos, R Egg … (2006)
A great variety of neurological diseases require investigation of cerebrospinal fluid (CSF) to prove the diagnosis or to rule out relevant differential diagnoses. The objectives were to evaluate the theoretical background and provide guidelines for clinical use in routine CSF analysis including total protein, albumin, immunoglobulins, glucose, lactate, cell count, cytological staining, and investigation of infectious CSF. The methods included a Systematic Medline search for the above-mentioned variables and review of appropriate publications by one or more of the task force members. Grading of evidence and recommendations was based on consensus by all task force members. It is recommended that CSF should be analysed immediately after collection. If storage is needed 12 ml of CSF should be partitioned into three to four sterile tubes. Albumin CSF/serum ratio (Qalb) should be preferred to total protein measurement and normal upper limits should be related to patients' age. Elevated Qalb is a non-specific finding but occurs mainly in bacterial, cryptococcal, and tuberculous meningitis, leptomingeal metastases as well as acute and chronic demyelinating polyneuropathies. Pathological decrease of the CSF/serum glucose ratio or increased lactate concentration indicates bacterial or fungal meningitis or leptomeningeal metastases. Intrathecal immunoglobulin G synthesis is best demonstrated by isoelectric focusing followed by specific staining. Cellular morphology (cytological staining) should be evaluated whenever pleocytosis is found or leptomeningeal metastases or pathological bleeding is suspected. Computed tomography-negative intrathecal bleeding should be investigated by bilirubin detection.
- Record: found
- Abstract: found
- Article: not found
Crossing the barrier: oxysterols as cholesterol transporters and metabolic modulators in the brain.
I Björkhem (2006)
A normal brain function requires constant levels of cholesterol, and the need for constancy seems to be higher here than in any other organ. Nature has met this need by isolation of brain cholesterol by a highly efficient blood-brain barrier. As a low synthesis of cholesterol is present in the brain, a mechanism for compensatory elimination is required. A decade ago we made the unexpected finding that the favoured mechanism for this involves conversion into 24S-hydroxycholesterol, followed by diffusion over the blood-brain barrier. Recent studies by us and others on this new pathway have given new insights into the mechanisms by which cholesterol homeostasis is maintained in the brain. We recently demonstrated a flux of another oxygenated product of cholesterol, 27-hydroxycholesterol, in the opposite direction. The latter flux may be important for neurodegeneration, and may be the link between hypercholesterolaemia and Alzheimer's disease. An overview of the above studies is presented and the possibility that the cholesterol 24S-hydroxylase in the brain may be important for memory and learning and that it may be a new drug target is discussed.
- Record: found
- Abstract: found
- Article: found
Oxysterols in the pathogenesis of major chronic diseases☆
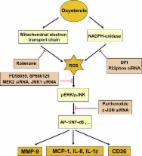
Introduction In order to degrade cholesterol to more polar compounds, which are more easily disposable by the cell, an oxygen function, such as a hydroxyl, epoxide or ketone group, is introduced into the sterol nucleus or side chain. The resulting 27-carbon intermediates or end-products of cholesterol metabolism are called oxysterols [1]. Of interest in pathophysiology, these compounds in general show a biochemical reactivity that is one or even two orders of magnitude higher than that of the parent compound. Further, due to the additional oxygen function, oxysterols are readily able to cross lipophilic membranes, unlike cholesterol [2,3]. Thus, if their production in cells and tissues and/or their introduction with dietary animal fat are excessive, oxysterols could indeed contribute to the pathogenesis of various disease processes. As schematically illustrated in Table 1, oxysterols may be originally present in food containing animal fat, but they are chiefly generated during food storing and cooking. The oxysterols produced within cells and tissues are undoubtedly more important in human pathology. With regard to endogenous sources of oxysterols, oxidation of the sterol ring of cholesterol is almost always non-enzymatic, leading to compounds like 7-ketocholesterol (7-K), 7β-hydroxycholesterol (7β-OH), 5α,6α-epoxycholesterol (α-EPOX) and 5β,6β-epoxycholesterol (β-EPOX). Conversely, 7α-hydroxycholesterol (7α-OH) is produced via an enzymatic mechanism, as are all oxysterols deriving from the oxidation of the side chain, e.g. 24-hydroxycholesterol (24-OH) and 27-hydroxycholesterol (27-OH) [4,5]. Pro-inflammatory effect of oxysterols The oxysterols of interest in pathophysiology, of which the main ones are listed above, are unanimously recognized as strongly enhancing inflammatory reactions, by inducing both expression and synthesis of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin-6 (IL-6), chemokines such as interleukin-8 (IL-8), monocyte chemotactic protein-1 (MCP-1) and monocyte inflammatory protein-1β (MIP-1β), and adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1) and E-selectin [6]. Notably, the expression of these and other inflammatory mediators is closely dependent on the activity of nuclear factor-κB (NF-κB), a transcription peptide demonstrated to be strongly up-regulated by oxysterols, through activation of the protein kinase C-extracellular signaling-regulated kinase 1/2 (PKC-ERK1/2) pathway [7]. This does not mean that other transcription factors, for example activator protein-1 (AP-1), are not also important in modulating the expression of inflammation-related cytokines. Very recently, a mixture of oxysterols similar to that detectable in human atherosclerotic lesions has been confirmed to up-regulate AP-1, and not only NF-κB, in human promonocytic cells [8]. Inflammation is undoubtedly a major driving force for the progression and complication of major chronic diseases. The recruitment and activation of phagocytes, characteristic of inflammatory reactions, lead to an increased steady-state level of radical and non-radical reactive oxygen species (ROS), that in turn amplify inflammation, giving rise to a vicious circle that sustains and expands the inflammatory process. The first important consequence of this event is that any inflammatory process determines a shift of the biochemical redox equilibrium in cells and tissues towards an excess of oxidative reactions, and oxidative stress is the main endogenous mechanism of non-enzymatic generation of oxysterols. As a second notable consequence, the reciprocal ability of inflammation and oxysterols to up-regulate one another, tends to amplify the overall inflammatory process: i n f l a m m a t i o n → o x i d a t i v e s t r e s s → c h o l e s t e r o l o x i d a t i o n → i n f l a m m a t i o n This scheme is probably too simple, since the actual interaction between inflammation and cholesterol is most likely further complicated, in disease processes for which hypercholesterolemia, i.e. a perturbation of cholesterol metabolism, is a risk factor. These include atherosclerosis, but also Alzheimer's and Parkinson's diseases, inflammatory bowel diseases and colon cancer, alcoholic and non-alcoholic steatosis and steatohepatitis. In addition, at least in the brain, the enzymatic production of oxysterols is remarkable already under physiological conditions [9]. Cholesterol oxidation in the pathogenesis of atherosclerosis Hypercholesterolemia has long been related to the pathogenesis of atherosclerosis, but the actual mechanism by which cholesterol favors both the initiation and the progression of this disease is still debated. The epidemiologic evidence supporting cholesterol as a primary risk factor of cardiovascular diseases has not been validated thus far by available biochemical data, which clearly indicate cholesterol as a poorly reactive molecule. Conversely, especially in the last 10–15 years, a number of experimental studies have consistently shown that cholesterol oxidation products, in particular oxysterols, possess a much higher biochemical reactivity than that of the parent compound [4]. This evidence prompted several research groups to focus on the potential modulation of atherosclerotic disease, by the oxysterols that have been detected in the blood of hypercholesterolemic individuals and in human atheromas [10,11]. Oxysterols, like other lipid oxidation products, have been shown to contribute to the endothelial cell dysfunction that characterizes the onset of the atheromasic plaque [12]. In particular, the oxysterols 7α-OH, 7β-OH and 7-K have been demonstrated to induce a clear inflammatory phenotype in human endothelial cells [13], and 7-K induces foam cell formation [14]. With regard to the molecular signaling underlying these effects of oxysterols, the central role played by PKCδ and by ERK1/2 pathway was conclusively demonstrated through small interfering RNA (siRNA) technology [15]. More recently, together with metalloproteases implicated in the pathogenesis of major human diseases, the potential impact of oxysterols on atherosclerotic plaque erosion and rupture has received increasing attention. In this connection, the evidence of over-expression of matrix metalloproteinase-9 (MMP-9) in freshly isolated human monocytes, activated in vitro by incubation with phorbol myristate acetate, is of particular interest. This treatment is known to enhance NADPH oxidase isoform 2 (Nox2) in phagocytes. Induction of MMP-9, an independent risk factor for atherothrombotic events [16], was then shown to depend on stimulation of Nox2, since inactivation of the latter enzyme with a selective inhibitor, apocynin, prevented up-regulation of this metalloproteinase [17]. Indeed, some years previously, mouse peritoneal macrophages, incubated in the presence of a mixture of oxysterols of biological relevance, were shown to markedly increase ROS intracellular steady-state, by activating Nox2 [18]. These results were later reproduced in human promonocytic cells challenged with either an oxysterol mixture or 27-OH or 7α-OH given alone [8], thus confirming that certain oxysterols may also exert a direct and marked pro-oxidant effect. Notably, oxysterols have been shown to enhance MMP-9 levels and activity in human cells of the macrophage lineage, through the induction of Nox2 activity and, consequently, of ROS production. However, they did not modify levels of the tissue inhibitors of metalloproteinases, TIMP-1 and TIMP-2 [8]. In an earlier study, a similar increase in the MMP-9/TIMPs ratio was observed in human advanced atherosclerotic lesions causing stable angina or an acute coronary syndrome, but the molecules responsible for this imbalance have not yet been investigated [19]. Most likely, other lipid oxidation products generated within oxidized low density lipoprotein (LDL) may also exert similar effects, which may not necessarily be limited to MMP-9. Be that as it may, extensive oxysterol-mediated degradation of extracellular matrix components of the fibrotic cap of advanced atheromas would render them unstable and prone to rupture. Fig. 1 shows the main signaling pathways that are up-regulated by the oxysterols detectable in atherosclerotic plaques and leading to increased expression and levels of MMP-9, inflammatory cytokines (e.g. MCP-1, IL-8, IL-1β) and scavenger receptor CD36. Compounds such as 7α-OH and 27-OH activate the PKCδ isoform as well as the classic isoforms, involving a G-protein and the phospholipase C-dependent cascade [8,15]. Activation of the PKC family isoenzymes drives oxysterol-generated signals through the mitogen-activated protein kinase (MAPK) pathway, with marked up-regulation of ERK and c-Jun N-terminal (JNK) kinases, but apparently without modulating p38 kinase [8]. Another PKC-driven effect is a sustained up-regulation of Nox2, with consequent excessive production of ROS. ROS overproduction, due to Nox2 but also to impaired mitochondrial respiration, certainly contributes to mediating and amplifying oxysterol-induced signaling through the cell, with eventual activation of redox-sensitive transcription factors. Among these transcription peptides, experimental data obtained thus far clearly indicate the involvement of NF-κB and AP-1. The involvement of these enzymes and transcription peptides has been demonstrated in human promonocytic cells, using specific inhibitors and/or siRNAs [8]. Increasing data, obtained in our laboratory, suggest that the same signaling pathway might be up-regulated by cholesterol oxides in other cell types, such as neuronal cells and epithelial colonic cells. Altered cholesterol metabolism, oxysterols and neurodegenerative diseases As has been said, hypercholesterolemia has long been considered to act as the primary risk factor for developing Alzheimer's disease and, possibly, Parkinson's disease [20,21]. Further, impaired brain cholesterol distribution and metabolism has more recently been pointed to as likely involved in the pathogenesis of these and other neurodegenerative diseases [22,23]. Oxysterols could play a very important role in brain pathophysiology since, unlike cholesterol, they can permeate the blood–brain barrier [24]. Although this fact means that the brain can eliminate excess amounts of these oxidation products, it could conversely allow toxic amounts of oxysterols, present in the blood stream, to accumulate in the brain. The latter event has been definitely demonstrated for 27-OH [25]. To date, the oxysterols most widely considered to be potentially implicated in the pathogenesis of neurodegenerative disease processes are 24-OH and 27-OH, both of enzymatic origin [4,5] and good ligands of the liver X receptors (LXRs) [26]. This class of receptors is now recognized as very important, although their multifaceted physiological role has yet to be fully elucidated [27]. The oxysterol 24-OH is produced by cholesterol 24-hydroxylase (cytochrome P450-46A1), a fairly specific brain enzyme, essentially (at least in the normal brain) localized in neuronal cells. To date it has been considered to exert a protective action in the central nervous system, mainly it may favor the efflux of excess cholesterol from the brain to the blood [9]. However, the same oxysterol has recently been shown to markedly potentiate both the pro-apoptotic and the pro-necrogenic effects exerted by 1–42 amyloid-β (Aβ1–42) peptide on two different human neuronal cell lines [28] but also on human dental pulp progenitor cells differentiated into neuronal-like cells [29]. In those in vitro experimental models, 24-OH (1 μM) appeared to interact with Aβ1–42 by strongly increasing intracellular ROS steady-state [28]. This evidence fits the widely-held opinion that Aβ needs to form oligomers to induce neurotoxicity, and that the latter process is probably enhanced by oxidative redox imbalance [30]. In this connection, the histochemical observation of a prevalent localization of cytochrome P450-46A1 in the vicinity of amyloid deposits, in autoptic brain samples from Alzheimer's patients, is of interest [31]; oxidative stress/lipid peroxidation appear implicated in this disease from its early onset [32,33]. Unlike cytochrome P450-46A1, cytochrome P450-27A1, the enzyme that generates 27-OH, is present in various tissues [9]. Accumulation in the brain of 27-OH, either deriving from the blood in hypercholesterolemic subjects, or generated in situ, has been reported to be potentially toxic, and to be associated with Alzheimer's disease [34]. In the various in vitro tests performed in our laboratory, 27-OH often displayed lower neurotoxicity than did 24-OH [28]. Most likely, the actual effect of an abnormal accumulation of 24-OH or 27-OH depends on a series of factors, i.e. localization in the brain, co-presence of oxidative redox imbalance, iron accumulation, relative antioxidant depletion, etc. Moreover, a study of some years ago reported that the Aβ peptide and, to a lesser extent, also the amyloid precursor protein, induced generation of 7β-OH [35]. In a very recent retrospective study carried out in cardiovascular patients with evidence of cerebrovascular disease, higher plasma levels of 24-OH and a greater ratio of 24-OH/27-OH are associated with the development of incident cognitive impairment over eight years of follow-up [36]. These findings heavily implicate a perturbation of cholesterol metabolism in the early stages of neurodegenerative disease process, including Alzheimer's disease development. Recent lipidomics analysis on human Parkinson's disease brains found an increased amount of oxysterols selectively localized in the visual cortex. Interestingly, 24-OH and 27-OH were not the only two oxysterols increased, the levels of 7α-OH, 7β-OH, β-EPOX and 7K also being increased about two fold in Parkinson's disease brains versus control brains [37]. This study, in our opinion, has the merit of expanding the viewpoint when examining oxysterols potentially implicated in the pathogenesis of neurodegenerative diseases. This recent approach, considering also oxysterols of non-enzymatic origin, appears to be valid, because of the recognized involvement of oxidative stress in both the initiation and the progression of brain degenerative disease processes [32,33]. Oxysterols and inflammatory bowel diseases The strong pro-inflammatory and pro-apoptotic properties exerted by excessive levels of several oxysterols have recently been conclusively demonstrated; this has provided new insight into the mechanisms of progression of human disease processes that are sustained by chronic inflammatory reactions. Ulcerative colitis and Crohn's disease, two frequent chronic diseases of the gut classified within the group of inflammatory bowel diseases (IBD), could easily be favored and worsened by a cholesterol-rich dietary regimen, typical of the Western diet. Bearing this in mind, Biasi and colleagues showed that a mixture of oxysterols stemming from the heating of dietary cholesterol, comprising 7-K, 7α-OH, 7β-OH, α-EPOX and β-EPOX, led differentiated human epithelial colonic cells (CaCo-2 cells) to apoptotic death. Specifically, caspase-3 activation after cell challenge, in the presence of the oxysterol mixture, was found to be 30–40% higher than in untreated cells [38]. The observed pro-apoptotic effect was prevented when up-regulation of Nox1 was inhibited; Nox1 is the intestinal isoform of NADPH oxidase, and its activation increases intracellular ROS steady-state [38]. The same oxysterol mixture (30 μM), but also one of its component, i.e. 7β-OH (4.4 μM), also exhibited a strong pro-inflammatory effect on differentiated CaCo-2 cells, causing the rapid over-expression of several inflammatory cytokines, namely IL-1α, IL-6, IL-8, IL-23, MCP-1, transforming growth factor β1 (TGFβ1), but apparently not TNF-α nor IL-10 [39]. Whether involved or not in the onset of IBD, dietary oxysterols most likely contribute to its amplification and further development. In addition, up-regulation of TGFβ1 in human colonic cells by oxysterols might induce the selective growth and development of atypical cell clones, resistant to the differentiating and pro-apoptotic effects of this cytokine, thus favoring colon cancer progression [40]. Oxysterols in degenerative changes of the retina The potential implication of oxysterols in age-related macular degeneration (AMD), in particular those generated by non-enzymatic reactions like 7-K, has recently been systematically analyzed and reviewed [41]. The strong pro-oxidant, pro-inflammatory, and pro-apoptotic properties of relatively high amounts of 7-K have been confirmed in vitro, using human retinal ARPE.19 cells. Other active oxysterols are 7β-OH and 25-OH. Of interest, as for epithelial colonic cells [39], the inflammatory cytokine most strongly enhanced by oxysterols in retinal cells was IL-8, while no significant effect was detected with regard to TNF-α and IL-10 synthesis and secretion [42]. An increasing bulk of data thus suggest that an altered cholesterol metabolism, with abnormal accumulation of oxysterols, could also be an important pathogenetic factor in the case of AMD. Further, since the main source of lipids in the retina appears to be plasma LDL [41], which is very rich in cholesterol, hypercholesterolemia might promote the expression of AMD. In this connection, a cholesterol-rich diet has been demonstrated to cause AMD-like retinal degeneration in the rabbit [43]. Of note, since accumulation of Aβ also occurs in AMD, it now appears likely that the peptide's neurotoxicity is potentiated by oxysterols, although this is yet to be confirmed. Oxysterols and disease processes characterized by oxidative redox imbalance An overview of recent literature on the main biochemical effects and potential pathogenic roles of various oxysterols suggests that these cholesterol metabolites might contribute, perhaps even significantly, to the expression and development of all disease processes promoted by inflammation and oxidative stress. It is thus likely that oxysterols will in the near future be given much more consideration when investigating the mechanisms leading to the onset and the progression of diseases such as obesity, diabetes mellitus, alcoholic and non-alcoholic fatty liver diseases, hemochromatosis, and chronic obstructive pulmonary diseases. Briefly, it is worth remembering that oxysterols deriving from the oxidation of the side chain of cholesterol (e.g. 24-OH, 27-OH, 25-OH) are very good ligands of LXRs and farnesoid X receptors (FXRs), whose hyperactivation is now recognized as implicated in both diabetes mellitus and non-alcoholic fatty liver disease [44], among others. Further, cholesterol synthesis has been shown to increase in non-alcoholic fatty liver disease [45], a disease in the progression of which oxidative stress, a significant non-enzymatic source of oxysterols, is certainly a primary factor [5]. Finally, future research on “enzymatic oxysterols” is particularly promising with regard to the interaction of these molecules with nuclear receptors, while the study of “oxidative stress oxysterols” could provide new insights in the pathogenesis of major chronic diseases in humans. Conclusive remarks As a final point, it appears important to stress that, although the majority of experimental studies have challenged cells or tissues with individual oxysterols, in the living organism oxysterols are present as mixtures, whether of endogenous or of exogenous origin. Like many other molecules with biochemical and/or pharmacological effects, oxysterols interact with one another in such a way as to modulate the effect of such mixtures. Quenching reactions among different components of oxysterol mixtures have been reported with regard to the role of oxysterols in atherosclerosis [46] and IBD progression [39]. Complex interactions of oxysterols likely occur in vivo, thus in vitro results concerning biochemical properties of pathologic amounts of individual oxysterols must be carefully weighed up and, where possible, accompanied and/or followed by analogous experiments with biologically compatible oxysterol mixtures.
Author and article information
Journal
Title:
Journal of Alzheimer's Disease
Abbreviated Title:
JAD
Publisher:
IOS Press
ISSN
(Print):
13872877
ISSN
(Electronic):
18758908
Publication date Created:
January
24 2017
Publication date
(Print):
January
24 2017
Volume: 56
Issue: 2
Pages: 825-833
Affiliations
[1
]Molecular Markers Laboratory, IRCCS Istituto Centro San Giovanni di Dio Fatebenefratelli,
Brescia, Italy
Article
DOI: 10.3233/JAD-160930
PubMed ID: 27983556
SO-VID: e8f7a36a-a484-4490-98b8-1abe9ddf8816
Copyright © ©
2017
History
Data availability: