- Record: found
- Abstract: found
- Article: found
Iodide modulates protein damage induced by the inflammation-associated heme enzyme myeloperoxidase
Read this article at
Abstract
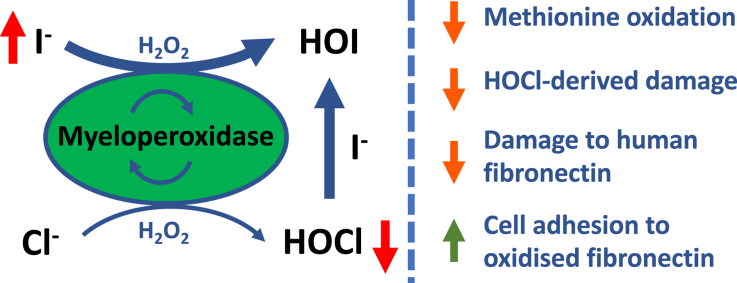
Iodide ions (I −) are an essential dietary mineral, and crucial for mental and physical development, fertility and thyroid function. I − is also a high affinity substrate for the heme enzyme myeloperoxidase (MPO), which is involved in bacterial cell killing during the immune response, and also host tissue damage during inflammation. In the presence of H 2O 2 and Cl −, MPO generates the powerful oxidant hypochlorous acid (HOCl), with excessive formation of this species linked to multiple inflammatory diseases. In this study, we have examined the hypothesis that elevated levels of I − would decrease HOCl formation and thereby protein damage induced by a MPO/Cl −/H 2O 2 system, by acting as a competitive substrate. The presence of increasing I − concentrations (0.1–10 μM; i.e. within the range readily achievable by oral supplementation in humans), decreased damage to both model proteins and extracellular matrix components as assessed by gross structural changes (SDS-PAGE), antibody recognition of parent and modified protein epitopes (ELISA), and quantification of both parent amino acid loss (UPLC) and formation of the HOCl-biomarker 3-chlorotyrosine (LC-MS) (reduced by ca. 50% at 10 μM I −). Elevated levels of I − ( > 1 μM) also protected against functional changes as assessed by a decreased loss of adhesion (eg. 40% vs. < 22% with >1 μM I −) of primary human coronary artery endothelial cells (HCAECs), to MPO-modified human plasma fibronectin. These data indicate that low micromolar concentrations of I −, which can be readily achieved in humans and are readily tolerated, may afford protection against cell and tissue damage induced by MPO.
Graphical abstract
Highlights
-
•
Iodide ions (I-) are an essential dietary mineral and critical to biological function.
-
•
Myeloperoxidase (MPO)-derived oxidants are bactericidal, but also damage host tissue.
-
•
Levels can be readily elevated in humans and animals by supplementation.
-
•
Is a high affinity substrate for MPO and acts as a competitive substrate.
-
•
Decreases MPO-mediated damage to model proteins and extracellular matrix species.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Myeloperoxidase and cardiovascular disease.
- Record: found
- Abstract: found
- Article: not found