- Record: found
- Abstract: found
- Article: found
Metagenomic Snapshots of Viral Components in Guinean Bats

Read this article at
Abstract
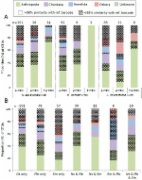
To prevent the emergence of zoonotic infectious diseases and reduce their epidemic potential, we need to understand their origins in nature. Bats in the order Chiroptera are widely distributed worldwide and are natural reservoirs of prominent zoonotic viruses, including Nipah virus, Marburg virus, and possibly SARS-CoV-2. In this study, we applied unbiased metagenomic and metatranscriptomic approaches to decipher the virosphere of frugivorous and insectivorous bat species captured in Guéckédou, Guinea, the epicenter of the West African Ebola virus disease epidemic in 2013–2016. Our study provides a snapshot of the viral diversity present in these bat species, with several novel viruses reported for the first time in bats, as well as some bat viruses closely related to known human or animal pathogens. In addition, analysis of Mops condylurus genomic DNA samples revealed the presence of an Ebola virus nucleoprotein (NP)-derived pseudogene inserted in its genome. These findings provide insight into the evolutionary traits of several virus families in bats and add evidence that nonretroviral integrated RNA viruses (NIRVs) derived from filoviruses may be common in bat genomes.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0.
- Record: found
- Abstract: found
- Article: not found