- Record: found
- Abstract: found
- Article: found
Detachment of Hexokinase II From Mitochondria Promotes Collateral Sensitivity in Multidrug Resistant Chronic Myeloid Leukemia Cells
Read this article at
Abstract
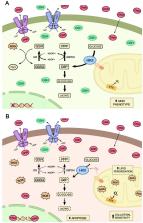
Chronic Myeloid Leukemia is a neoplastic disease characterized by the abnormal expansion of hematopoietic cells with compromised functions. Leukemic cells often display a multidrug resistance phenotype, enabling them to evade a number of structurally unrelated cytotoxic compounds. One of those mechanisms relies on the high expression of efflux transporters, such as the ABC proteins, whose activity depends on the hydrolysis of ATP to reduce intracellular drug accumulation. In the present work, we employed a well-known erythroleukemia cell line, K562, and a multidrug resistant derivative cell, FEPS, to evaluate how hexokinase II, a key regulator for the rate-limiting step glycolysis, contributes to the establishment of the multidrug resistance phenotype. We found that multidrug resistant cells primarily resort to glycolysis to generate ATP. Clotrimazole reduced the expression of mitochondrial hexokinase II, which destabilized bioenergetic parameters such as reactive oxygen species production, ATP, and glutathione levels on multidrug resistant cells. This impaired the activity of ABCC1, leading to increased drug accumulation and cell death. In summary, we propose that decoupling of hexokinase II from the mitochondria emerges as a promising strategy to generate collateral sensitivity and aid in the management of chronic myeloid leukemia in chemotherapy-refractory patients.
Related collections
Most cited references80
- Record: found
- Abstract: found
- Article: not found
Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method.
- Record: found
- Abstract: found
- Article: not found
The pentose phosphate pathway and cancer.
- Record: found
- Abstract: found
- Article: not found