- Record: found
- Abstract: found
- Article: found
Cyr61 promotes epithelial-mesenchymal transition and tumor metastasis of osteosarcoma by Raf-1/MEK/ERK/Elk-1/TWIST-1 signaling pathway
Read this article at
Abstract
Background
Osteosarcoma is the most common primary malignant tumor in children and young adults, and its treatment requires effective therapeutic approaches because of a high mortality rate for lung metastasis. Epithelial to mesenchymal transition (EMT) has received considerable attention as a conceptual paradigm for explaining the invasive and metastatic behavior during cancer progression. The cysteine-rich angiogenic inducer 61 (Cyr61) gene, a member of the CCN gene family, is responsible for the secretion of Cyr61, a matrix-associated protein that is involved in several cellular functions. A previous study showed that Cyr61 expression is related to osteosarcoma progression. In addition, Cyr61 could promote cell migration and metastasis in osteosarcoma. However, discussions on the molecular mechanism involved in Cyr61-regulated metastasis in osteosarcoma is poorly discussed.
Results
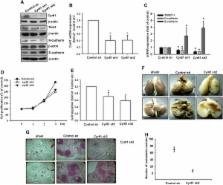
We determined that the expression level of Cyr61 induced cell migration ability in osteosarcoma cells. The Cyr61 protein promoted the mesenchymal transition of osteosarcoma cells by upregulating mesenchymal markers (TWIST-1 and N-cadherin) and inhibiting the epithelial marker (E-cadherin). Moreover, the Cyr61-induced cell migration was mediated by EMT. The Cyr61 protein elicited a signaling cascade that included αvβ5 integrin, Raf-1, mitogen-activated protein kinase (MEK), extracellular signal-regulated kinase (ERK), and Elk-1. The reagent or gene knockdown of these signaling proteins could inhibit Cyr61-promoted EMT in osteosarcoma. Finally, the knockdown of Cyr61 expression obviously inhibited cell migration and repressed mesenchymal phenotypes, reducing lung metastasis.
Related collections
Most cited references49
- Record: found
- Abstract: found
- Article: not found
Epithelial-mesenchymal transitions in development and disease.
- Record: found
- Abstract: found
- Article: not found
The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion.
- Record: found
- Abstract: found
- Article: not found