- Record: found
- Abstract: found
- Article: not found
Platelets secrete stromal cell–derived factor 1α and recruit bone marrow–derived progenitor cells to arterial thrombi in vivo
Read this article at
Abstract
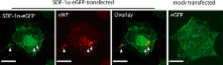
The accumulation of smooth muscle and endothelial cells is essential for remodeling and repair of injured blood vessel walls. Bone marrow–derived progenitor cells have been implicated in vascular repair and remodeling; however, the mechanisms underlying their recruitment to the site of injury remain elusive. Here, using real-time in vivo fluorescence microscopy, we show that platelets provide the critical signal that recruits CD34 + bone marrow cells and c-Kit + Sca-1 + Lin − bone marrow–derived progenitor cells to sites of vascular injury. Correspondingly, specific inhibition of platelet adhesion virtually abrogated the accumulation of both CD34 + and c-Kit + Sca-1 + Lin − bone marrow–derived progenitor cells at sites of endothelial disruption. Binding of bone marrow cells to platelets involves both P-selectin and GPIIb integrin on platelets. Unexpectedly, we found that activated platelets secrete the chemokine SDF-1α, thereby supporting further primary adhesion and migration of progenitor cells. These findings establish the platelet as a major player in the initiation of vascular remodeling, a process of fundamental importance for vascular repair and pathological remodeling after vascular injury.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell.
- Record: found
- Abstract: found
- Article: not found
Platelets in inflammation and thrombosis.
- Record: found
- Abstract: found
- Article: not found
