- Record: found
- Abstract: found
- Article: found
Src and ROCK Kinases Differentially Regulate Mineralization of Human Osteosarcoma Saos-2 Cells
Read this article at
Abstract
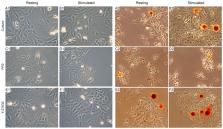
Osteoblasts initiate bone mineralization by releasing matrix vesicles (MVs) into the extracellular matrix (ECM). MVs promote the nucleation process of apatite formation from Ca 2+ and P i in their lumen and bud from the microvilli of osteoblasts during bone development. Tissue non-specific alkaline phosphatase (TNAP) as well as annexins (among them, AnxA6) are abundant proteins in MVs that are engaged in mineralization. In addition, sarcoma proto-oncogene tyrosine-protein (Src) kinase and Rho-associated coiled-coil (ROCK) kinases, which are involved in vesicular transport, may also regulate the mineralization process. Upon stimulation in osteogenic medium containing 50 μg/mL of ascorbic acid (AA) and 7.5 mM of β-glycerophosphate (β-GP), human osteosarcoma Saos-2 cells initiated mineralization, as evidenced by Alizarin Red-S (AR-S) staining, TNAP activity, and the partial translocation of AnxA6 from cytoplasm to the plasma membrane. The addition of 4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo [3,4-d] pyrimidine (PP2), which is an inhibitor of Src kinase, significantly inhibited the mineralization process when evaluated by the above criteria. In contrast, the addition of (R)-(+)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexane carboxamide hydrochloride (Y-27632), which is an inhibitor of ROCK kinase, did not affect significantly the mineralization induced in stimulated Saos-2 cells as denoted by AR-S and TNAP activity. In conclusion, mineralization by human osteosarcoma Saos-2 cells seems to be differently regulated by Src and ROCK kinases.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Rapid flow cytofluorometric analysis of mammalian cell cycle by propidium iodide staining
- Record: found
- Abstract: found
- Article: not found
Rapidly forming apatitic mineral in an osteoblastic cell line (UMR 106-01 BSP).
- Record: found
- Abstract: found
- Article: not found