- Record: found
- Abstract: found
- Article: not found
Dual inhibition of EGFR and mTOR pathways in small cell lung cancer
Read this article at
Abstract
Background:
In this report we investigated the combination of epidermal growth factor receptor (EGFR) and mammalian target of rapamycin (mTOR) pathway inhibition as a possible new therapeutic strategy for small cell lung cancer (SCLC).
Methods:
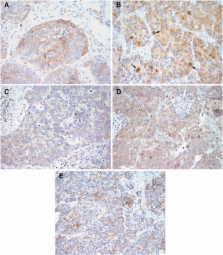
EGFR, p-AKT, p-ERK, p-mTOR and p-p70s6K protein expressions were studied by immunohistochemistry in 107 small cell lung carcinomas and correlated with clinicopathological parameters. Cells of SCLC were treated with erlotinib±RAD001 and analysed for cell viability, proliferation, autophagy, and pathway regulation.
Results:
Epidermal growth factor receptor, p-AKT, p-ERK, p-mTOR, and p-p70s6K were expressed in 37, 24, 13, 55 and 91% of the tumour specimens of all SCLC patients, respectively, and were not associated with disease-free or overall survival. The expression of EGFR was lower in neoadjuvant-treated patients ( P=0.038); mTOR pathway activation was higher in the early stages of disease ( P=0.048). Coexpression of EGFR/p-mTOR/p-p70s6K was observed in 28% of all patients . EGFR immunoreactivity was associated with p-ERK and p-mTOR expression ( P=0.02 and P=0.0001); p-mTOR immunoreactivity was associated with p-p70s6K expression ( P=0.001). Tumour cells comprised a functional EGFR, no activating mutations in exons 18–21, and resistance to RAD001 monotherapy. We found synergistic effects of erlotinib and RAD001 combination therapy on the molecular level, cell viability, proliferation and autophagy.
Related collections
Most cited references27
- Record: found
- Abstract: not found
- Article: not found
The TOR pathway: a target for cancer therapy.
- Record: found
- Abstract: found
- Article: not found
Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs.
- Record: found
- Abstract: found
- Article: not found
