- Record: found
- Abstract: found
- Article: not found
MAPK pathway activation in pilocytic astrocytoma
Read this article at
Abstract
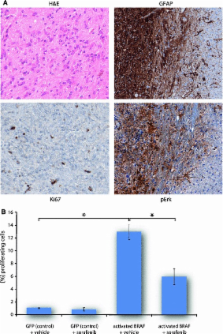
Pilocytic astrocytoma (PA) is the most common tumor of the pediatric central nervous system (CNS). A body of research over recent years has demonstrated a key role for mitogen-activated protein kinase (MAPK) pathway signaling in the development and behavior of PAs. Several mechanisms lead to activation of this pathway in PA, mostly in a mutually exclusive manner, with constitutive BRAF kinase activation subsequent to gene fusion being the most frequent. The high specificity of this fusion to PA when compared with other CNS tumors has diagnostic utility. In addition, the frequency of alteration of this key pathway provides an opportunity for molecularly targeted therapy in this tumor. Here, we review the current knowledge on mechanisms of MAPK activation in PA and some of the downstream consequences of this activation, which are now starting to be elucidated both in vitro and in vivo, as well as clinical considerations and possible future directions.
Related collections
Most cited references95
- Record: found
- Abstract: found
- Article: not found
Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a.
- Record: found
- Abstract: found
- Article: not found
Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas.
- Record: found
- Abstract: found
- Article: not found
