- Record: found
- Abstract: found
- Article: found
Preparation of Biodegradable Oligo(lactide)s-Grafted Dextran Nanogels for Efficient Drug Delivery by Controlling Intracellular Traffic
Read this article at
Abstract
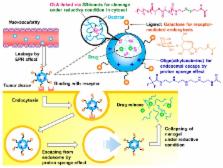
Nanogels, nanometer-sized hydrogel particles, have great potential as drug delivery carriers. To achieve effective drug delivery to the active sites in a cell, control of intracellular traffic is important. In this study, we prepared nanogels composed of dextran with oligolactide (OLA) chains attached via disulfide bonds (Dex- g-SS-OLA) that collapse under the reductive conditions of the cytosol to achieve efficient drug delivery. In addition, we introduced galactose (Gal) residues on the nanogels, to enhance cellular uptake by receptor-mediated endocytosis, and secondary oligo-amine (tetraethylenepentamine) groups, to aid in escape from endosomes via proton sponge effects. The obtained Dex- g-SS-OLA with attached Gal residues and tetraethylenepentamine (EI 4) groups, EI 4/Gal-Dex- g-SS-OLA, formed a nanogel with a hydrodynamic diameter of ca. 203 nm in phosphate-buffered solution. The collapse of the EI 4/Gal-Dex- g-SS-OLA nanogels under reductive conditions was confirmed by a decrease in the hydrodynamic diameter in the presence of reductive agents. The specific uptake of the hydrogels into HepG2 cells and their intercellular behavior were investigated by flow cytometry and confocal laser scanning microscopy using fluorescence dye-labeled nanogels. Escape from the endosome and subsequent collapse in the cytosol of the EI 4/Gal-Dex- g-SS-OLA were observed. These biodegradable nanogels that collapse under reductive conditions in the cytosol should have great potential as efficient drug carriers in, for example, cancer chemotherapy.
Related collections
Most cited references33
- Record: found
- Abstract: not found
- Article: not found
Lectins: Carbohydrate-Specific Proteins That Mediate Cellular Recognition.
- Record: found
- Abstract: found
- Article: not found
Intelligent polymeric micelles from functional poly(ethylene glycol)-poly(amino acid) block copolymers.
- Record: found
- Abstract: found
- Article: not found