- Record: found
- Abstract: found
- Article: found
Signal Transducer and Activator of Transcription (STATs) Proteins in Cancer and Inflammation: Functions and Therapeutic Implication
Read this article at
Abstract
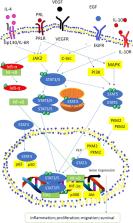
Signal Transducer and Activator of Transcription (STAT) pathway is connected upstream with Janus kinases (JAK) family protein and capable of integrating inputs from different signaling pathways. Each family member plays unique functions in signal transduction and crucial in mediating cellular responses to different kind of cytokines. STAT family members notably STAT3 and STAT5 have been involved in cancer progression whereas STAT1 plays opposite role by suppressing tumor growth. Persistent STAT3/5 activation is known to promote chronic inflammation, which increases susceptibility of healthy cells to carcinogenesis. Here, we review the role of STATs in cancers and inflammation while discussing current therapeutic implications in different cancers and test models, especially the delivery of STAT3/5 targeting siRNA using nanoparticulate delivery system.
Related collections
Most cited references148
- Record: found
- Abstract: found
- Article: not found
IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity.
- Record: found
- Abstract: found
- Article: not found