- Record: found
- Abstract: found
- Article: found
Paternal age in rhesus macaques is positively associated with germline mutation accumulation but not with measures of offspring sociability
Read this article at
Abstract
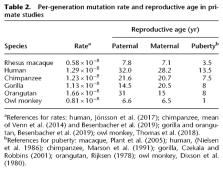
Mutation is the ultimate source of all genetic novelty and the cause of heritable genetic disorders. Mutational burden has been linked to complex disease, including neurodevelopmental disorders such as schizophrenia and autism. The rate of mutation is a fundamental genomic parameter and direct estimates of this parameter have been enabled by accurate comparisons of whole-genome sequences between parents and offspring. Studies in humans have revealed that the paternal age at conception explains most of the variation in mutation rate: Each additional year of paternal age in humans leads to approximately 1.5 additional inherited mutations. Here, we present an estimate of the de novo mutation rate in the rhesus macaque ( Macaca mulatta) using whole-genome sequence data from 32 individuals in four large pedigrees. We estimated an average mutation rate of 0.58 × 10 −8 per base pair per generation (at an average parental age of 7.5 yr), much lower than found in direct estimates from great apes. As in humans, older macaque fathers transmit more mutations to their offspring, increasing the per generation mutation rate by 4.27 × 10 −10 per base pair per year. We found that the rate of mutation accumulation after puberty is similar between macaques and humans, but that a smaller number of mutations accumulate before puberty in macaques. We additionally investigated the role of paternal age on offspring sociability, a proxy for normal neurodevelopment, by studying 203 male macaques in large social groups.
Related collections
Most cited references75
- Record: found
- Abstract: found
- Article: not found
From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline.
- Record: found
- Abstract: not found
- Article: not found
Observational Study of Behavior: Sampling Methods
- Record: found
- Abstract: found
- Article: not found