- Record: found
- Abstract: found
- Article: found
Short‐term outcomes of a prospective multicenter phase II trial of total neoadjuvant therapy for locally advanced rectal cancer in Japan (ENSEMBLE‐1)

Read this article at
Abstract
Aim
To evaluate the feasibility and safety of total neoadjuvant therapy (TNT) in patients with locally advanced rectal cancer (LARC) in Japan.
Methods
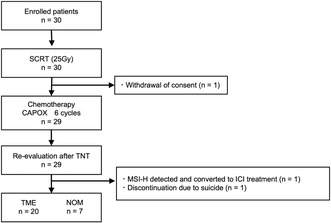
This prospective, multicenter, open‐label, single‐arm phase II trial was conducted at five institutions. The key eligibility criteria were age ≥ 20 years, LARC within 12 cm from the anal verge, and cT3‐4N0M0 or TanyN+M0 at the time of diagnosis that enabled curative resection. Preoperative short‐course radiation therapy (SCRT) 5 Gy × 5 days (total 25 Gy) + CAPOX (six courses) followed by total mesorectum excision (TME) was the treatment protocol. Non‐operative management (NOM) was allowed if clinical complete response (cCR) was obtained in the preoperative evaluation. The primary endpoint was the pathological complete response (pCR) rate.
Results
Thirty patients (male, n = 26; female, n = 4; median age, 62.5 [44–74] years; cT [T2, n = 1; T3, n = 25; T4, n = 4]; cN [N0, n = 13; N1, n = 13; N2, n = 4]) were enrolled. The final analysis included 30 patients in total. The completion rates were 100% for SCRT and 83% for CAPOX. TME and NOM were performed in 20 and seven patients, respectively. pCR was observed in six patients (30% [95% CI 14.0%–50.8%]). The primary endpoint was met. pCR+cCR was observed in 13 (43.3%) patients. There were no treatment‐related deaths. Grade ≥3 (CTCAE ver. 5.0) adverse events (≥20%), including diarrhea (23.3%) and neutropenia (23.3%). The median follow‐up period was 15.6 (10.5–22.8) months, with no recurrence or regrowth in NOM.
Abstract
This is the first phase 2 clinical trial conducted by a multicenter to investigate the feasibility and safety of total neoadjuvant therapy (TNT) for patients with locally advanced rectal cancer (LARC) in Japan. Pathological complete response (pCR) and pCR + cCR were observed in 30% and 43.3% of patients, respectively.
Related collections
Most cited references22
- Record: found
- Abstract: found
- Article: not found
Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries

- Record: found
- Abstract: found
- Article: found