- Record: found
- Abstract: found
- Article: found
Regulation of Wnt signaling by nociceptive input in animal models
Read this article at
Abstract
Background
Central sensitization-associated synaptic plasticity in the spinal cord dorsal horn (SCDH) critically contributes to the development of chronic pain, but understanding of the underlying molecular pathways is still incomplete. Emerging evidence suggests that Wnt signaling plays a crucial role in regulation of synaptic plasticity. Little is known about the potential function of the Wnt signaling cascades in chronic pain development.
Results
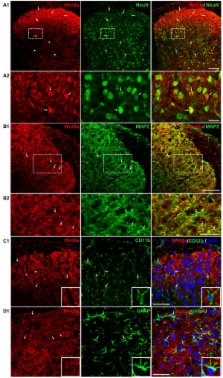
Fluorescent immunostaining results indicate that β-catenin, an essential protein in the canonical Wnt signaling pathway, is expressed in the superficial layers of the mouse SCDH with enrichment at synapses in lamina II. In addition, Wnt3a, a prototypic Wnt ligand that activates the canonical pathway, is also enriched in the superficial layers. Immunoblotting analysis indicates that both Wnt3a a β-catenin are up-regulated in the SCDH of various mouse pain models created by hind-paw injection of capsaicin, intrathecal (i.t.) injection of HIV-gp120 protein or spinal nerve ligation (SNL). Furthermore, Wnt5a, a prototypic Wnt ligand for non-canonical pathways, and its receptor Ror2 are also up-regulated in the SCDH of these models.
Related collections
Most cited references68
- Record: found
- Abstract: not found
- Article: not found
Ethical guidelines for investigations of experimental pain in conscious animals.
- Record: found
- Abstract: found
- Article: not found
An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat.
- Record: found
- Abstract: found
- Article: not found