- Record: found
- Abstract: found
- Article: found
Molecular docking studies of coumarin hybrids as potential acetylcholinesterase, butyrylcholinesterase, monoamine oxidase A/B and β-amyloid inhibitors for Alzheimer’s disease
Read this article at
Abstract
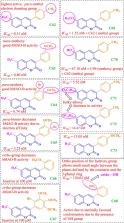
Coumarins are the phytochemicals, which belong to the family of benzopyrone, that display interesting pharmacological properties. Several natural, synthetic and semisynthetic coumarin derivatives have been discovered in decades for their applicability as lead structures as drugs. Coumarin based conjugates have been described as potential AChE, BuChE, MAO and β-amyloid inhibitors. Therefore, the objective of this review is to focus on the construction of these pharmacologically important coumarin analogues with anti-Alzheimer’s activities, highlight their docking studies and structure–activity relationships based on their substitution pattern with respect to the selected positions on the chromen ring by emphasising on the research reports conducted in between year 1968 to 2017.

Related collections
Most cited references100
- Record: found
- Abstract: found
- Article: not found
Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein.
- Record: found
- Abstract: not found
- Article: not found
Acetylcholinesterase: enzyme structure, reaction dynamics, and virtual transition states
- Record: found
- Abstract: found
- Article: not found