- Record: found
- Abstract: found
- Article: not found
High-density lipoprotein induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through sphingosine kinase-2
Read this article at
Abstract
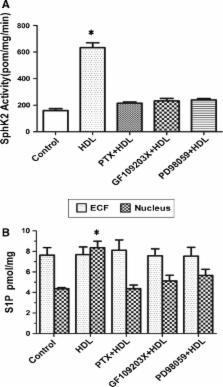
High-density lipoprotein (HDL) has a significant cardioprotective effects. HDL induces cyclooxygenase-2 (COX-2) expression and prostacyclin I-2 (PGI-2) release in vascular endothelial cells, which contributes to its anti-atherogenic effects. However, the underlying mechanisms are not fully understood. In the present study, we observed that HDL-stimulated COX-2 expression and PGI-2 production in human umbilical vein endothelial cells (HUVECs) in a time- and dose-dependent manner. These effects triggered by HDL were inhibited by pertussis toxin (PTX), protein kinase C (PKC) inhibitor GF109203X, and ERK inhibitor PD98059, suggesting that Gαi/Gαo-coupled GPCR, PKC, and ERK pathways are involved in HDL-induced COX-2/PGI-2 activation. More importantly, we found that silencing of sphingosine kinase 2 (SphK-2) also blocked HDL-induced COX-2/PGI-2 activation. In addition, HDL-activated SphK-2 phosphorylation accompanied by increased S1P level in the nucleus. Our ChIP data demonstrated that SphK-2 is associated with CREB at the COX-2 promoter region. Collectively, these results indicate that HDL induces COX-2 expression and PGI-2 release in endothelial cells through activation of PKC, ERK1/2, and SphK-2 pathways. These findings implicate a novel mechanism underlying anti-atherothrombotic effects of HDL.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Endothelial and antithrombotic actions of HDL.
- Record: found
- Abstract: found
- Article: not found
Erythrocytes store and release sphingosine 1-phosphate in blood.
- Record: found
- Abstract: not found
- Article: not found
