- Record: found
- Abstract: found
- Article: found
“Sweet Flavonoids”: Glycosidase-Catalyzed Modifications
Read this article at
Abstract
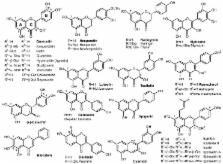
Natural flavonoids, especially in their glycosylated forms, are the most abundant phenolic compounds found in plants, fruit, and vegetables. They exhibit a large variety of beneficial physiological effects, which makes them generally interesting in a broad spectrum of scientific areas. In this review, we focus on recent advances in the modifications of the glycosidic parts of various flavonoids employing glycosidases, covering both selective trimming of the sugar moieties and glycosylation of flavonoid aglycones by natural and mutant glycosidases. Glycosylation of flavonoids strongly enhances their water solubility and thus increases their bioavailability. Antioxidant and most biological activities are usually less pronounced in glycosides, but some specific bioactivities are enhanced. The presence of l-rhamnose (6-deoxy-α- l-mannopyranose) in rhamnosides, rutinosides (rutin, hesperidin) and neohesperidosides (naringin) plays an important role in properties of flavonoid glycosides, which can be considered as “pro-drugs”. The natural hydrolytic activity of glycosidases is widely employed in biotechnological deglycosylation processes producing respective aglycones or partially deglycosylated flavonoids. Moreover, deglycosylation is quite commonly used in the food industry aiming at the improvement of sensoric properties of beverages such as debittering of citrus juices or enhancement of wine aromas. Therefore, natural and mutant glycosidases are excellent tools for modifications of flavonoid glycosides.
Related collections
Most cited references110
- Record: found
- Abstract: found
- Article: not found
The flavonoid quercetin in disease prevention and therapy: facts and fancies.
- Record: found
- Abstract: not found
- Article: not found
STEREOCHEMISTRY AND THE MECHANISM OF ENZYMATIC REACTIONS
- Record: found
- Abstract: found
- Article: not found