- Record: found
- Abstract: found
- Article: not found
Ataxin-3 Is a Multivalent Ligand for the Parkin Ubl Domain
Read this article at
Abstract

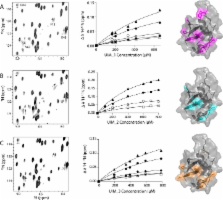
The ubiquitin signaling pathway consists of hundreds of enzymes that are tightly regulated for the maintenance of cell homeostasis. Parkin is an E3 ubiquitin ligase responsible for conjugating ubiquitin onto a substrate protein, which itself can be ubiquitinated. Ataxin-3 performs the opposing function as a deubiquitinating enzyme that can remove ubiquitin from parkin. In this work, we have identified the mechanism of interaction between the ubiquitin-like (Ubl) domain from parkin and three C-terminal ubiquitin-interacting motifs (UIMs) in ataxin-3. 1H– 15N heteronuclear single-quantum coherence titration experiments revealed that there are weak direct interactions between all three individual UIM regions of ataxin-3 and the Ubl domain. Each UIM utilizes the exposed β-grasp surface of the Ubl domain centered around the I44 patch that did not vary in the residues involved or the surface size as a function of the number of ataxin-3 UIMs involved. Further, the apparent dissociation constant for ataxin-3 decreased as a function of the number of UIM regions used in experiments. A global multisite fit of the nuclear magnetic resonance titration data, based on three identical binding ligands, resulted in a K D of 669 ± 62 μM for each site. Our observations support a multivalent ligand binding mechanism employed by the parkin Ubl domain to recruit multiple UIM regions in ataxin-3 and provide insight into how these two proteins function together in ubiquitination–deubiquitination pathways.
Related collections
Most cited references32
- Record: found
- Abstract: found
- Article: not found
Structure of parkin reveals mechanisms for ubiquitin ligase activation.
- Record: found
- Abstract: found
- Article: not found
Autoregulation of Parkin activity through its ubiquitin-like domain.
- Record: found
- Abstract: found
- Article: not found
