- Record: found
- Abstract: found
- Article: found
Rapid response of thyroid eye disease, peripheral edema, and acropathy to teprotumumab infusion
Read this article at
Abstract
Purpose
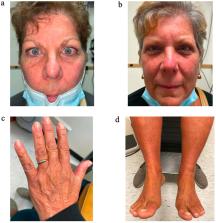
We present a case of rapid improvement in symptoms of thyroid eye disease and amelioration of worsening peripheral edema and acropathy with infusion of teprotumumab, a monoclonal antibody targeting the insulin-like growth factor-1 receptor.
Observations
A 66 year old female with history of Hashimoto thyroiditis developed progressive thyroid eye disease (TED), peripheral edema, and acropathy attributable to acute Graves disease. Her signs and symptoms, refractory to oral steroid and diuretic therapy, rapidly improved following a standard dosing regimen of teprotumumab (one infusion 10 mg/kg then seven infusions 20 mg/kg) to resolution.
Conclusions & importance
Teprotumumab, a monoclonal antibody targeting the insulin-like growth factor-1 receptor, is the first medication approved by the FDA for use in TED. Teprotumumab may contribute to the treatment of extraocular manifestations of Graves disease, chief among these peripheral soft tissue manifestations.
Related collections
Most cited references9

- Record: found
- Abstract: found
- Article: found
Teprotumumab, an insulin-like growth factor-1 receptor antagonist antibody, in the treatment of active thyroid eye disease: a focus on proptosis
- Record: found
- Abstract: found
- Article: not found
