- Record: found
- Abstract: found
- Article: found
Pseudorabies Virus Infection Alters Neuronal Activity and Connectivity In Vitro
Read this article at
Abstract
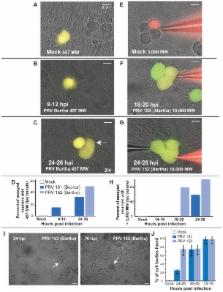
Alpha-herpesviruses, including human herpes simplex virus 1 & 2, varicella zoster virus and the swine pseudorabies virus (PRV), infect the peripheral nervous system of their hosts. Symptoms of infection often include itching, numbness, or pain indicative of altered neurological function. To determine if there is an in vitro electrophysiological correlate to these characteristic in vivo symptoms, we infected cultured rat sympathetic neurons with well-characterized strains of PRV known to produce virulent or attenuated symptoms in animals. Whole-cell patch clamp recordings were made at various times after infection. By 8 hours of infection with virulent PRV, action potential (AP) firing rates increased substantially and were accompanied by hyperpolarized resting membrane potentials and spikelet-like events. Coincident with the increase in AP firing rate, adjacent neurons exhibited coupled firing events, first with AP-spikelets and later with near identical resting membrane potentials and AP firing. Small fusion pores between adjacent cell bodies formed early after infection as demonstrated by transfer of the low molecular weight dye, Lucifer Yellow. Later, larger pores formed as demonstrated by transfer of high molecular weight Texas red-dextran conjugates between infected cells. Further evidence for viral-induced fusion pores was obtained by infecting neurons with a viral mutant defective for glycoprotein B, a component of the viral membrane fusion complex. These infected neurons were essentially identical to mock infected neurons: no increased AP firing, no spikelet-like events, and no electrical or dye transfer. Infection with PRV Bartha, an attenuated circuit-tracing strain delayed, but did not eliminate the increased neuronal activity and coupling events. We suggest that formation of fusion pores between infected neurons results in electrical coupling and elevated firing rates, and that these processes may contribute to the altered neural function seen in PRV-infected animals.
Author Summary
Alpha-herpesviruses, including human herpes simplex virus 1 and 2, varicella zoster virus and swine pseudorabies virus, infect the peripheral nervous system of their hosts. Symptoms often include itching, numbness, or pain. Understanding of the physiological basis for these characteristic sensory and motor anomalies remains limited. To provide more insight, we examined the electrical activity of infected neurons in culture. We used pseudorabies virus (PRV) because infected animals show strain dependent, and often, dramatic symptoms (violent scratching and self mutilation). We found that infected neurons exhibited increased action potential (AP) rates. Infected neurons also became electrically coupled, proceeding from small molecular weight dye sharing and coupled activity to large molecular weight dye sharing and complete electrical synchrony. Late in infection, cell bodies fused to form syncytia. When neurons were infected with a virulent strain that could not express glycoprotein B, a member of the viral membrane fusion complex, AP rates were not increased. Changes in electrical connectivity and dye transfer were not observed, and syncytia did not occur. These results suggest that infection induced elevated activity and electrical coupling result from virally induced membrane fusion and may underlie neurological symptoms observed during infection.
Related collections
Most cited references67
- Record: found
- Abstract: found
- Article: not found
Genital herpes.
- Record: found
- Abstract: not found
- Article: not found
Information processing in the axon.
- Record: found
- Abstract: found
- Article: not found