- Record: found
- Abstract: found
- Article: found
Chromosome Segregation Is Biased by Kinetochore Size
Read this article at
Summary
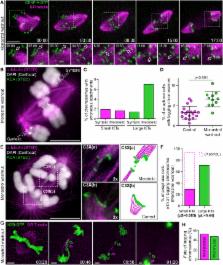
Chromosome missegregation during mitosis or meiosis is a hallmark of cancer and the main cause of prenatal death in humans. The gain or loss of specific chromosomes is thought to be random, with cell viability being essentially determined by selection. Several established pathways including centrosome amplification, sister-chromatid cohesion defects, or a compromised spindle assembly checkpoint can lead to chromosome missegregation. However, how specific intrinsic features of the kinetochore—the critical chromosomal interface with spindle microtubules—impact chromosome segregation remains poorly understood. Here we used the unique cytological attributes of female Indian muntjac, the mammal with the lowest known chromosome number (2n = 6), to characterize and track individual chromosomes with distinct kinetochore size throughout mitosis. We show that centromere and kinetochore functional layers scale proportionally with centromere size. Measurement of intra-kinetochore distances, serial-section electron microscopy, and RNAi against key kinetochore proteins confirmed a standard structural and functional organization of the Indian muntjac kinetochores and revealed that microtubule binding capacity scales with kinetochore size. Surprisingly, we found that chromosome segregation in this species is not random. Chromosomes with larger kinetochores bi-oriented more efficiently and showed a 2-fold bias to congress to the equator in a motor-independent manner. Despite robust correction mechanisms during unperturbed mitosis, chromosomes with larger kinetochores were also strongly biased to establish erroneous merotelic attachments and missegregate during anaphase. This bias was impervious to the experimental attenuation of polar ejection forces on chromosome arms by RNAi against the chromokinesin Kif4a. Thus, kinetochore size is an important determinant of chromosome segregation fidelity.
Graphical Abstract
Highlights
-
•
Centromere/kinetochore functional layers scale proportionally with centromere size
-
•
Kinetochore microtubule binding capacity scales with kinetochore size
-
•
Chromosome congression and bi-orientation are biased by kinetochore size
-
•
Error formation leading to chromosome missegregation is biased by kinetochore size
Abstract
Drpic et al. use fibroblasts from female Indian muntjac, the mammal with the lowest known chromosome number (2n = 6), to show that chromosome congression and bi-orientation are biased by kinetochore size. Chromosomes with larger kinetochores are also biased to establish erroneous merotelic attachments and missegregate during anaphase. Thus, kinetochore size is an important determinant of chromosome segregation fidelity.
Related collections
Most cited references56
- Record: found
- Abstract: found
- Article: not found
Fluorogenic probes for live-cell imaging of the cytoskeleton.
- Record: found
- Abstract: found
- Article: not found
Aurora B phosphorylates spatially distinct targets to differentially regulate the kinetochore-microtubule interface.
- Record: found
- Abstract: found
- Article: not found
