- Record: found
- Abstract: found
- Article: found
Urolithin B suppresses osteoclastogenesis via inhibiting RANKL‐induced signalling pathways and attenuating ROS activities
Read this article at
Abstract
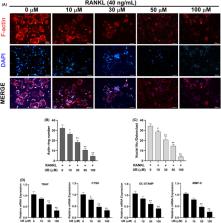
Osteoporosis (OP) has severely affected human health, which is characterized by abnormal differentiation of osteoclasts. Urolithin B (UB), as a potential natural drug, has been reported to exhibit numerous biological activities including antioxidant and anti‐inflammatory but its effects on OP, especially on RANKL‐stimulated osteoclast formation and activation, are still understood. In our study, we have demonstrated for the first time that UB inhibits RANKL‐induced osteoclast differentiation and explored its potential mechanisms of action. The RAW264.7 cells were cultured and induced with RANKL followed by UB treatment. Then, the effects of UB on mature osteoclast differentiation were evaluated by counting tartrate‐resistant acid phosphatase (TRAP)‐positive multinucleated cells and F‐actin ring analysis. Moreover, the effects of UB on RANKL‐induced reactive oxygen species (ROS) were measured by 2′, 7′‐dichlorodihydrofluorescein diacetate (DCFH‐DA) staining. Further, we explored the potential mechanisms of these downregulation effects by performing Western blotting and quantitative RT‐PCR examination. We found that UB represses osteoclastogenesis, F‐actin belts formation, osteoclast‐specific gene expressions and ROS activity in a time‐ and concentration‐dependent manner. Mechanistically, UB attenuates intracellular ROS levels by upregulation of Nrf2 and other ROS scavenging enzymes activation. Furthermore, UB also inhibited RANKL‐induced NF‐κB, MAPK and Akt signalling pathway and suppressed expression of c‐Fos and nuclear factor of activated T cells 1 (NFATc1), which is the master transcription factor of osteoclast differentiation. Taken together, our findings confirm that UB is a polyphenolic compound that can be a potential therapeutic treatment for osteoclast‐related bone diseases such as osteoporosis.
Related collections
Most cited references48
- Record: found
- Abstract: found
- Article: not found
Osteoclast differentiation and activation.
- Record: found
- Abstract: found
- Article: not found
Pathogenesis of osteoporosis: concepts, conflicts, and prospects.
- Record: found
- Abstract: found
- Article: not found