- Record: found
- Abstract: found
- Article: found
Tanshinol ameliorates CCl 4-induced liver fibrosis in rats through the regulation of Nrf2/HO-1 and NF-κB/IκBα signaling pathway

Abstract
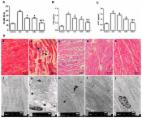
Tanshinol, a water-soluble component isolated from Salvia miltiorrhiza Bunge, has a variety of biological activities involving anti-fibrotic effect. However, the exact role and the underlying mechanisms remain largely unclear. This study mainly focused on the anti-hepatic fibrotic activities and mechanisms of tanshinol on carbon tetrachloride (CCl 4)-induced liver fibrosis in rats via anti-oxidative and anti-inflammation pathways. The rats were divided into 4 groups as follows: control, model, tanshinol 20 mg/kg, and tanshinol 40 mg/kg. Except for the control group, CCl 4 was used to induce liver fibrosis processing for 8 weeks, meanwhile rats in tanshinol groups were intraperitoneally injected with additional tanshinol. Control group simultaneously received the same volumes of olive oil and saline. The potentially protective effect and mechanisms of tanshinol on liver fibrosis in rats were evaluated. The serum levels of alanine aminotransferase, aspartate aminotransferase, and total bilirubin were obviously lower in the tanshinol treatment groups related to model group. Compared with the model group, the levels of hyaluronic acid, type IV collagen, Laminin (LN), and procollagen III peptide (PIIIP) in serum were significantly decreased after tanshinol treatment. Furthermore, tanshinol could regulate Nrf2/HO-1 signaling pathway and increase the level of superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px), and also decrease the level of malondialdehyde (MDA) to against damage induced by oxidative stress. Simultaneously tanshinol could regulate nuclear factor kappa B signaling pathway to inhibit expression of inflammation factors, including transforming growth factor-β, tumor necrosis factor-α, Cox-2, interleukin-1β, and interleukin-6. In summary, our research demonstrated that tanshinol has protective effect on CCl 4-induced liver fibrosis via inhibiting oxidative stress and inflammation, which may be associated with the regulation of nuclear factor erythroid2-related factor 2/hemeoxygenase-a and nuclear factor kappa B/inhibitor of kappa B alpha signaling pathways.
Most cited references31

- Record: found
- Abstract: found
- Article: found
Mesenchymal stem cell therapy for liver fibrosis
- Record: found
- Abstract: found
- Article: found
Paeonol and danshensu combination attenuates apoptosis in myocardial infarcted rats by inhibiting oxidative stress: Roles of Nrf2/HO-1 and PI3K/Akt pathway
- Record: found
- Abstract: found
- Article: not found