- Record: found
- Abstract: found
- Article: found
Relaxin gene delivery mitigates liver metastasis and synergizes with check point therapy
Read this article at
Abstract
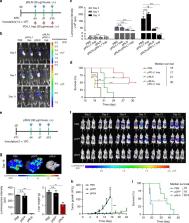
Activated hepatic stellate cell (aHSC)-mediated liver fibrosis is essential to the development of liver metastasis. Here, we discover intra-hepatic scale-up of relaxin (RLN, an anti-fibrotic peptide) in response to fibrosis along with the upregulation of its primary receptor (RXFP1) on aHSCs. The elevated expression of RLN serves as a natural regulator to deactivate aHSCs and resolve liver fibrosis. Therefore, we hypothesize this endogenous liver fibrosis repair mechanism can be leveraged for liver metastasis treatment via enforced RLN expression. To validate the therapeutic potential, we utilize aminoethyl anisamide-conjugated lipid-calcium-phosphate nanoparticles to deliver plasmid DNA encoding RLN. The nanoparticles preferentially target metastatic tumor cells and aHSCs within the metastatic lesion and convert them as an in situ RLN depot. Expressed RLN reverses the stromal microenvironment, which makes it unfavorable for established liver metastasis to grow. In colorectal, pancreatic, and breast cancer liver metastasis models, we confirm the RLN gene therapy results in significant inhibition of metastatic progression and prolongs survival. In addition, enforced RLN expression reactivates intra-metastasis immune milieu. The combination of the RLN gene therapy with PD-L1 blockade immunotherapy further produces a synergistic anti-metastatic efficacy. Collectively, the targeted RLN gene therapy represents a highly efficient, safe, and versatile anti-metastatic modality, and is promising for clinical translation.
Abstract
Activated hepatic stellate cells are associated with fibrosis and liver metastases. Here, the authors identify an endogenous role of relaxin in regulating the activation of hepatic stellate cells and report nanoparticle-mediated relaxin gene therapy to mitigate fibrosis and liver metastasis.
Related collections
Most cited references59
- Record: found
- Abstract: found
- Article: not found
Immunological hallmarks of stromal cells in the tumour microenvironment.
- Record: found
- Abstract: found
- Article: not found
High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation.
- Record: found
- Abstract: found
- Article: not found