- Record: found
- Abstract: found
- Article: found
Atrial remodeling and atrial fibrillation in acquired forms of cardiovascular disease
Read this article at
Abstract
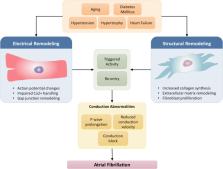
Atrial fibrillation (AF) is prevalent in common conditions and acquired forms of heart disease, including diabetes mellitus (DM), hypertension, cardiac hypertrophy, and heart failure. AF is also prevalent in aging. Although acquired heart disease is common in aging individuals, age is also an independent risk factor for AF. Importantly, not all individuals age at the same rate. Rather, individuals of the same chronological age can vary in health status from fit to frail. Frailty can be quantified using a frailty index, which can be used to assess heterogeneity in individuals of the same chronological age. AF is thought to occur in association with electrical remodeling due to changes in ion channel expression or function as well as structural remodeling due to fibrosis, myocyte hypertrophy, or adiposity. These forms of remodeling can lead to triggered activity and electrical re-entry, which are fundamental mechanisms of AF initiation and maintenance. Nevertheless, the underlying determinants of electrical and structural remodeling are distinct in different conditions and disease states. In this focused review, we consider the factors leading to atrial electrical and structural remodeling in human patients and animal models of acquired cardiovascular disease or associated risk factors. Our goal is to identify similarities and differences in the cellular and molecular bases for atrial electrical and structural remodeling in conditions including DM, hypertension, hypertrophy, heart failure, aging, and frailty.
Related collections
Most cited references145

- Record: found
- Abstract: found
- Article: found
A standard procedure for creating a frailty index
- Record: found
- Abstract: found
- Article: not found
Pathophysiology and treatment of type 2 diabetes: perspectives on the past, present, and future.
- Record: found
- Abstract: found
- Article: not found