- Record: found
- Abstract: found
- Article: found
Up-regulation of liver Pcsk9 gene expression as a possible cause of hypercholesterolemia in experimental chronic renal failure
Read this article at
Abstract
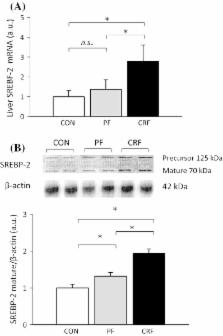
Dyslipidemia commonly present in patients with chronic kidney disease (CKD) has been recently linked to increased proprotein convertase subtilisin/kexin type 9 (PCSK9) serum concentration. We tested a hypothesis that increased liver PCSK9 biosynthesis could be partially responsible for the elevated circulating PCSK9 level, and subsequently contribute to hypercholesterolemia observed in subjects with CKD. Rat model of chronic renal failure (CRF) was used in the study. Animals underwent a 5/6 nephrectomy or a sham operation. Liver expression of Pcsk9, sterol regulatory element-binding transcription factor 2 ( Srebf- 2), and β- actin were quantified by real-time RT-PCR. Liver protein levels of PCSK9, LDL-receptor (LDL-R), and SREBF-2 were analyzed using Western blotting. Serum PCSK9 concentration was estimated by immunoassay. Rats with an experimental CRF as compared to pair-fed and control ones were characterized by: (a) an up-regulation of liver Pcsk9 and Srebf- 2 genes expression with parallel increase of serum PCSK9 concentration; (b) a decrease in liver LDL-R protein level, and (c) an increase of serum total and LDL-cholesterol concentrations. We also found significant correlations between serum creatinine and liver PCSK9 mRNA levels ( r = 0.88, p < 0.001) and between serum creatinine and circulating PCSK9 levels ( r = 0.73, p < 0.001). The results suggest that a rat model of CRF is associated with an increased liver Pcsk9 gene expression. The coordinated up-regulation of Pcsk9 and Srebf- 2 genes expression suggests that SREBF-2 may play a key role in regulation of Pcsk9 gene expression, circulating PCSK9 level, and hypercholesterolemia in experimental CRF.
Related collections
Most cited references18
- Record: found
- Abstract: found
- Article: not found
Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9.
- Record: found
- Abstract: found
- Article: not found
Hepatic PCSK9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c.
- Record: found
- Abstract: found
- Article: not found