- Record: found
- Abstract: found
- Article: found
Mucus and mucins in diseases of the intestinal and respiratory tracts
Read this article at
Abstract
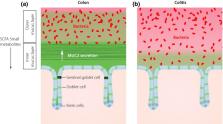
This review describes the organization and importance of mucus in the intestine and lungs in relation to the diseases cystic fibrosis ( CF), ulcerative colitis and chronic obstructive pulmonary disease (COPD). The inner surfaces of the body are protected by mucus built around polymeric glycoproteins called mucins. In the disease CF, the small intestinal mucus is in contrast the normal attached to the epithelium, explaining the intestinal problems at this disease. The inner of the two mucus layers of colon is normally impenetrable to bacteria, keeping the commensals away from and protecting the epithelium. This impenetrable property is dependent on the bacterial composition and the host diet, observations that can explain the increased incidence of inflammatory bowel diseases in the western world as bacteria reach the epithelial cells in active ulcerative colitis. The respiratory tract is normally cleared by thick mucus bundles that moved by the cilia sweep the epithelial surface. In CF, the bundles are nonmoving already at birth. Cholinergic stimulations stop the bundle movement explaining some of the beneficial effect of anticholinergic treatment in COPD. In this disease as well as in more developed CF, an attached mucus layer is formed. This mucus has features similar to the protective inner colon mucus and is by this able to separate bacteria from the epithelial surface. When formed in healthy individuals this mucus can be coughed up, but in chronically diseased lungs, bacteria colonizing the mucus will remain in the lungs and the resulting inflammation contribute to the destruction of the lungs.
Abstract
Content List ‐ 15th Key symposium ‐ “Innate immunity”.
Related collections
Most cited references33
- Record: found
- Abstract: found
- Article: not found
Energy contributions of volatile fatty acids from the gastrointestinal tract in various species.
- Record: found
- Abstract: found
- Article: not found
Insights into secondary metabolism from a global analysis of prokaryotic biosynthetic gene clusters.
- Record: found
- Abstract: found
- Article: not found