- Record: found
- Abstract: found
- Article: found
Affordability of medicines in the European Union
Read this article at
Abstract
Background
Medications and their prices are key issues for healthcare. Although access to medicines at affordable prices had been specified as a key objective of the European Health Policy, it seems that these goals have not been achieved. Therefore, we attempted an evaluation of affordability of selected medicines at full prices.
Methods
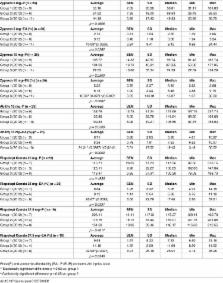
The analysis concerned 2012 and was conducted between 2013 and 2015 in all the European Union (EU) countries divided into 3 groups depending on the date of their accession to the EU. Finally, we considered 9 originators used in the treatment of schizophrenia and multiple sclerosis. Information on drug prices were collected from pharmacies. Participation in the study was voluntary and anonymous in order to avoid accusations of advertising. To evaluate affordability, several factors were used (e.g. minimum earnings and Gini coefficient). Due to unavailability in some countries, the exact number of analyzed medicines varies.
Results
Drug prices vary significantly between EU Member States. Almost eleven fold difference was observed between Germany (EUR 1451.17) and Croatia (EUR 132.77) in relation to Interferone beta-1a 22 μg. Generally, prices were the highest in Germany. The cheapest drugs were found in various countries but never in the poorest ones like Bulgaria or Romania. Discrepancies in wages were observed too (the smallest minimum wage was EUR 138.00 in Bulgaria and the highest EUR 1801.00 in Luxembourg). Full price of olanzapine 5mg, however, was higher in Bulgaria (EUR 64.53) than, for instance, in Belgium (EUR 37.26).
Conclusions
Analyzed medications are still unaffordable for many citizens of the EU. Besides, access to medicines is also impaired e.g. due to parallel trade. Unaffordability of medications may lead to the patients’ non-compliance and therefore to increased direct and indirect costs of treatment. Common European solutions are needed to achieve a real affordability and accessibility of medications.
Related collections
Most cited references23
- Record: found
- Abstract: found
- Article: not found
The global costs of schizophrenia.

- Record: found
- Abstract: found
- Article: found
Essential Medicines Are More Available than Other Medicines around the Globe
- Record: found
- Abstract: found
- Article: not found
