INTRODUCTION
Viruses that most commonly affect the human respiratory tract include rhinoviruses, respiratory syncytial viruses (RSVs), influenza, and coronaviruses (CoVs). RSV causes the most severe pathology in the lungs (bronchiolitis) with high mortality rates especially in children during their first year of life [1]. Bronchiolitis caused by RSV and rhinovirus is also associated with an increased risk of asthma developing [2]. Regular epidemics caused by influenza viruses result in 250,000-500,000 annual deaths worldwide [3, 4]. Until 2020, coronaviruses caused seasonal diseases, except for zoonoses caused by severe acute respiratory syndrome virus-1 (SARS-CoV-1) and Middle East respiratory syndrome (MERS) virus, but in March 2020, the first pandemic caused by the SARS-CoV-2 virus was declared. The disease named COVID-19 has led to the death of more than 6 million people worldwide as of March 2022 [5, 6].
Viruses are recognized by respiratory tract epithelial cells expressing pattern recognition receptors (PRRs). The main PRRs for the RNA viruses are the toll-like receptors (TLRs) 3, 7, 8 and the cytosolic RIG-like and NOD-like receptors. After penetrating the cell, the virus triggers an innate immune response accompanied by a massive release of pro-inflammatory cytokines aimed at suppressing viral replication [7]. The production of interferons (IFNs) I (α, β) and III (λ) types provides for the protection of neighboring uninfected epithelial cells from the virus infection. If the reproduction of the virus cannot be quickly suppressed, the immune cells produce pro-inflammatory cytokines (IL6, TNFα) that contribute to the recruitment of neutrophils and T cells into the respiratory tract, which eventually leads to the development of inflammation [8, 9, 10].
To date, the greatest threat is posed by COVID-19 with identified 5 phases of disease development, including the incubation period, symptom development, early inflammation, secondary infection, and multisystem inflammation syndrome (MIS) phases [11]. The incubation period can last from 2 to 14 days. An active innate immunity of a patient leads to the rapid elimination of the virus and an asymptomatic form of the disease, which occurs in 40-45% of cases [12, 13]. In cases with a less effective immune response, several symptoms develop including fever, cough, muscle pain, loss of smell, and gastrointestinal disorders. At the early inflammation stage, patients can be treated with direct-acting antiviral drugs. An increase in the severity of COVID-19 is more often observed in elderly patients and individuals with concomitant diseases. In these cases, an unbalanced immune response with low levels of IFNs type I production and excessive production of IL6 and TNFα prevents the rapid elimination of the virus [14-16]. These patients may develop hypoxemia, pulmonary pathology, cardiac dysfunction, renal failure, neurological manifestations, or multiple organ failure [17, 18]. At this phase (previously known as the cytokine storm), treatment with dexamethasone or drugs that suppress IL6 (monoclonal antibodies to the IL6 receptor), such as tocilizumab may attenuate the excess inflammatory response [19]. If left untreated, COVID-19 can progress to a secondary bacterial or fungal infection requiring antibacterial or antifungal therapy. A common outcome in COVID-19 is organizing pneumonia with damage to the bronchioles and alveoli, which is also common for RSV infection and influenza [20].
An unfavorable development of COVID-19 may result in MIS [21]. In this case, excessive dysfunctional reactions prevent the elimination of the virus and lead to severe consequences, including vasculitis, thromboembolic processes, and immune-mediated thrombocytopenia [22-24]. The level of IL6 at this stage can be 10-200 times higher than at the early inflammation phase [25].
In cases of severe COVID-19, IFNγ plays the leading role in the control of the cellular immune response to an infection by activating macrophages and enhancing antigen presentation as well as T-cell differentiation through the interaction with IFNγ receptors [26, 27]. IFNγ can directly transmit signals to epithelial cells by means of JAK/STAT signaling pathway, causing the expression of chemokines [28]. One of the main chemokines in cases of severe respiratory tract infections is IFNγ-inducible protein 10 (CXCL10) – its level in plasma and bronchoalveolar lavage (BAL) of patients correlates with the severity of the disease [29, 30]. Patients’ sera also contain CXCL2, IL8, and CXCL9 chemokines [31]. A high level of inflammatory cytokines leads to cell death, acute damage to tissues and organs, abnormal changes in vascular hemostasis, and, as a result, to multiple organ failure [32]. Inhibition of the individual branches of the immune response can limit the destructive effect of inflammation [33]. In cases with developing lung pathology and damage to the vasculature of the respiratory tract, it is necessary to reduce the virus-induced inflammation at all stages of the infectious process regardless of the timing of the start of treatment with antiviral agents.
XC221GI was originally developed as a treatment for an RSV infection in immunosuppressed patients and children. XC221GI showed the highest efficacy against RSV infection from a series of novel chemical compounds studied in the cotton rat model (recommended model for RSV infection studies). Here we present the results of our study on the anti-inflammatory activity of XC221GI in respiratory tract infections. The goal of this study is to determine if XC221GI can be used for the preventive treatment of RSV and SARS-CoV-2 infections. We have shown that XC221GI is effective against RSV infection in vitro in a continuous A549 epithelial cell line and in vivo in the cotton rat model. In addition, we demonstrated the effect of XC221GI on the induction of the chemokines CXCL10, CXCL9, and CXCL11 and neutrophil influx in the mouse model with the inflammation induced by the intranasal (i.n.) administration of IFNγ to animals.
MATERIALS AND METHODS
Preparation
According to the IUPAC nomenclature, XC221GI is (1-[2-(1-methylimidazol-4-yl)-ethyl] perhydroazin-2,6-dione) with a molar mass of 221.3 g/mol. The substance XC221GI was produced and released by Alven Laboratories s.r.o (Czech Republic) in accordance with the current European standards of Good Manufacturing Practice.
The effect of XC221GI on the production of IL6 and IL8 in RSV infected cells
The continuous cell line of human lung carcinoma (A549, ATCC) was cultivated in DMEM/F-12 medium (Gibco, USA) containing 2% glutamine and 10% fetal bovine serum at 37°C in a CO2 incubator (5%) at a humidity of 95%. The human RSV (strain A2, Influenza Reagent Resource, IRR, USA) was cultured in a continuous epithelial cell line MA-104. The experiment was conducted in an A549 cell line in a serum-free DMEM/F-12 medium. A virus-containing fluid was used for the analysis.
The solution of XC221GI was added to wells with a monolayer of A549 cells (previously washed with PBS) to final concentrations of 0.001 μg/ml, 0.01 μg/ml, 0.1 μg/ml, 1 μg/ml, and 3 μg/ml. Simultaneously, RSV was added with a multiplicity of infection (MOI) of 0.01. The total volume was adjusted with a culture medium to 500 μl/well. Addition of the drug was repeated in 24 h. A serum-free DMEM-F12 medium supplemented with 2% Gluta-Max culture (Gibco, USA) was used to dilute the substance and virus. Wells with RSV-infected cells without the addition of XC221GI served as a control for virus reproduction. Uninfected cells with the addition of the drug served as a negative control. The plates were incubated in a CO2 incubator at 37°C. At 48 h after infection, the culture medium was harvested and the concentrations of IL6 and IL8 were quantified using the commercial ELISA test systems Human IL6 and Human IL8/NAP-1 Platinum ELISA (eBioscience, USA).
The cytotoxic effect of XC221GI was assessed using a colorimetric microtetrazolium test using 3-(4,5-Di-methyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT). To determine the cytotoxic effect, XC221GI was added to plates with A549 cells at a concentration of 0.005–5000 μg/ml and incubated within 48 h at 37°C, 5% CO2, and 95% relative humidity with the repeated addition of the drug after 24 h.
Effect of XC221GI on lung pathology in cotton rats infected with RSV
Animals
The experiments were conducted at Sigmovir Biosystems, Inc. (Rockville, USA). Female cotton rats (Sigmodon hispidus) (aged 6 to 8 weeks, with a body weight of 70-100 g) were obtained from a colony of Sigmovir Biosystems, Inc. The animals were housed in large polycarbonate cages and provided with standard rodent chow and water ad libitum. The cotton rats used in the experiment were seronegative for foreign respiratory viruses and other common rodent pathogens. All of the experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee of the University of Maryland (Institutional Animal Care and Use Committee, IACUC). All of the animals were ear tagged with identification numbers. After infection with RSV, the animals were kept individually.
Infection of animals
Each animal received light anesthesia (isoflurane inhalation) before infection. RSV (A2 strain) was administered to cotton rats i.n. with a pipette at a dose of 5x105 plaque forming units (PFU).
Pathology score
The rats were divided into 3 groups (5 animals in each). Cotton rats infected with RSV and treated with XC221GI (20 mg/kg/day, administered with drinking water) orally (p.o.) in the course of 5 days starting 24 h after infection. The animals of the control group were infected with RSV and received drinking water without XC221GI; the animals of the intact group received phosphate buffered saline (PBS) instead of RSV and drinking water without the drug. On the 6th day after infection, the animals were sacrificed, the lungs were removed, and histological slides were prepared and stained with hematoxylin-eosin (H&E). Two parameters of lung pathology were assessed, including interstitial pneumonia (inflammatory cell infiltration and thickening of the alveolar walls) and alveolitis (cells in the alveolar spaces). Slides were blindly scored from 0 to 4 using the Sigmovir Biosystems, Inc. severity scale [34]. A semi-quantitative histological system for inflammation assessing included the number of interstitial and alveolar infiltrates: 0% – 0 points, 0-25% – 1 point, 25-50% – 2 points, 50-75% – 3 points, >75% – 4 points. The obtained scores were summed up [35]. The effect of XC221GI was evaluated by comparing a group of treated animals with a control group that received water instead of the drug.
The second experiment was conducted using cotton rats with experimental immunosuppression induced by the administration of cyclophosphamide (CY). CY was administered at a dose of 50 mg/kg daily over 3 weeks; right before infection, the dose was reduced to 37.5 mg/kg and then to 25 mg/kg on the day of infection and thereafter. In 24 h after the i.n. administration of 5x105 PFU RSV, the animals received XC221GI (20 mg/kg/day) p.o. with drinking water and were receiving this medication further over 9 days. RSV-infected animals given pure drinking water served as positive controls. Animals treated with PBS and pure drinking water served as negative controls. The lungs of rats were extracted on the 10th day after infection and the virus titer as well as the severity of histopathological changes in the lungs were assessed. Four slides stained with H&E, Duffy, PAS, or toluidine blue were prepared for each lung sample and used for the detailed pathology assessment (see the section Criteria for Pathology Evaluation in Cotton Rats with RSV Infection in Supplementary Material). Polymorphonuclear leukocytes were also differentiated and identified as neutrophilic and/or eosinophilic by Duffy histochemical staining (eosinophils stain). Preparations stained with toluidine blue were used to assess the number, distribution, and morphology of mast cells. Goblet cells were assessed using PAS-stained preparations. Mucosal cell hyperplasia/hypertrophy and bronchiolar epithelium hyperplasia were also assessed. The assessment scores were summed together to obtain a total histopathology score. The data are presented as the proportion of animals (%) with a total histopathological change score of 1 to 8 points for the peribronchiolitis assessment and 1 to 6 points for interstitial pneumonia and alveolitis assessment.
Titration of RSV in the lungs of animals
Lung tissues were weighed and homogenized in 3.0 ml HBSS buffer supplemented with 10% SPG (sucrose/phosphate/glutamate) buffer, clarified by centrifugation and diluted in EMEM medium. The HEp-2 monolayer was infected with tenfold virus dilutions in 24-well plates. After 1 h incubation at 37°C and 5% CO2, the wells were covered with a medium containing 0.75% methylcellulose. After 4 days of incubation, the top layer was removed and the cells were fixed with 0.1% crystal violet within 1 h, and then washed and air dried. The number of plaques was counted, and the virus titer was expressed in PFU per gram of lung tissue.
Effect of XC221GI on neutrophil recruitment and the production of chemokines CXCL9, CXCL10, and CXCL11 in mouse lungs
Animals
Male 10-week-old C57BL6 mice weighing 26.9±1.7 g were purchased from the nursery of the Andreevka branch of the Scientific Center for Biomedical Technologies (Andreevka, Moscow Region, Russia). The animals were kept in controlled habitat cages with standard pelleted food and water. All of the experiments with animals were approved by the Animal Ethics Committee of Pharminterprices LLC (Veterinary certificates No. 12896925029 from 1/11/2022, No. 12896967735 from 1/11/2022). Experimental procedures with animals were performed according to the Guidelines for the Care and Use of Laboratory Animals [36]. All of the procedures with animals were performed under anesthesia.
The administration of IFNγ to mice and the quantification of neutrophils and chemokines CXCL10, CXCL9, and CXCL11
Animals were divided into groups, respectively. After anesthesia, 30 μl (100 ng/μl) of the recombinant IFNγ (R&D systems, USA) were injected i.n. in the right nostril of animals and then they immediately received XC221GI once p.o. at doses of 0.3, 3, or 15 mg/kg, or the reference drug – dexamethasone (Bryntsalov-A, Russia) at a dose of 1 mg/kg. A group of control animals received sterile saline instead of the drug solution. The animals of the intact group only received saline without the drug and without IFNγ. In 8-12 h after treatment, the samples of BAL were collected from animals under terminal anesthesia. To do this, the trachea was excised, a plastic cannula was inserted, and the air space was washed with 1 ml of PBS preheated to 37°C. The absolute number of cells in BAL was determined using the Goryaev camera. BAL was centrifuged for 10 min at 200 g and smears were prepared from the cell sediment. Smears were fixed in methanol, stained according to the Romanovsky-Giemsa method, and then the number of neutrophils was counted. The level of chemokines CXCL10, CXCL9, and CXCL11 was also determined in BAL using the test systems IP-10 (CXCL10) Mouse ELISA Kit (Thermo Fisher Scientific, USA), MIG/CXCL9 Mouse ELISA Kit (R&D Systems, USA), and CXCL11/I- TAC DuoSet (R&D Systems, USA).
Statistical analysis
Statistical data analysis was performed using GraphPad Prism 9 software (USA). The data were presented as the arithmetic mean (M)±standard deviation (SD) or standard error of the mean (SEM). The statistical significance of differences between groups was assessed using One-way ANOVA followed by the Tukey test at a significance level of p<0.05.
RESULTS
Evaluation of the XC221GI effect on the in vitro expression of IL6 and IL8 in RSV infected cells
The concentration of early cytokines and chemokines IL6, IL8, and TNFα in the blood during a viral infection can serve as a marker of disease severity [37]. The concentrations increase significantly with hyperinflammation. IL6 plays a central role in severe SARS-CoV-2 infection [25, 38]. The more severe course of the disease was shown to correlate with increased concentrations of IL1β, IL1RA, IL6, IL7, IL8, G-CSF, and CXCL9 in patients with RSV infection [39-41].
We investigated the effect of XC221GI on the induction of IL6 and IL8 in the in vitro model of RSV infection. We showed that the addition of XC221GI to the A549 cells infected with RSV led to the suppression of the production of IL6 (by 55-85%) and IL8 (by 40%) within 48 h in a dose-dependent manner (Fig. 1 A, B). The 50% IL6 inhibition was achieved at the XC221GI dose of 27.6 nmol/l (6.1 ng/ml). The presence of XC221GI in uninfected A549 cells had no effect on the IL8 levels. The level of IL6 in uninfected cells was below the detection limit and remained below the detection limit after the administration of XC221GI.
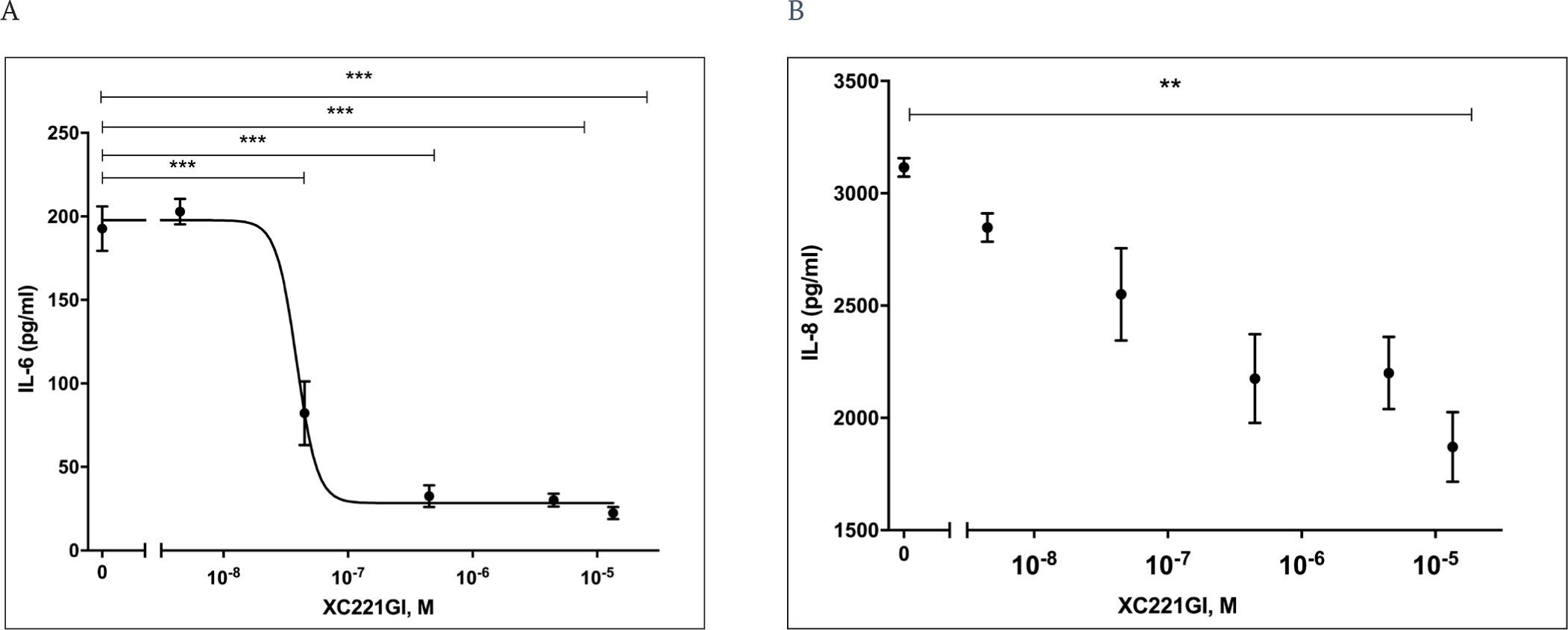
No cytotoxic effect of XC221GI in a concentration range up to 500 μg/ml was observed in A549 cells according to the MTT assay (data not shown).
Effect of XC221GI on the pathological changes in the lungs of cotton rats infected by RSV
The anti-inflammatory activity of XC221GI in viral lung injury caused by RSV was studied in a series of in vivo experiments in the cotton rat model. The investigational drug XC221GI was administrated daily (p.o., 20 mg/kg/day) to RSV-infected cotton rats with drinking water over 5 days starting 24 h after infection. The evaluation of the investigational drug activity was carried out on the 6th day when the pathology was the most pronounced.
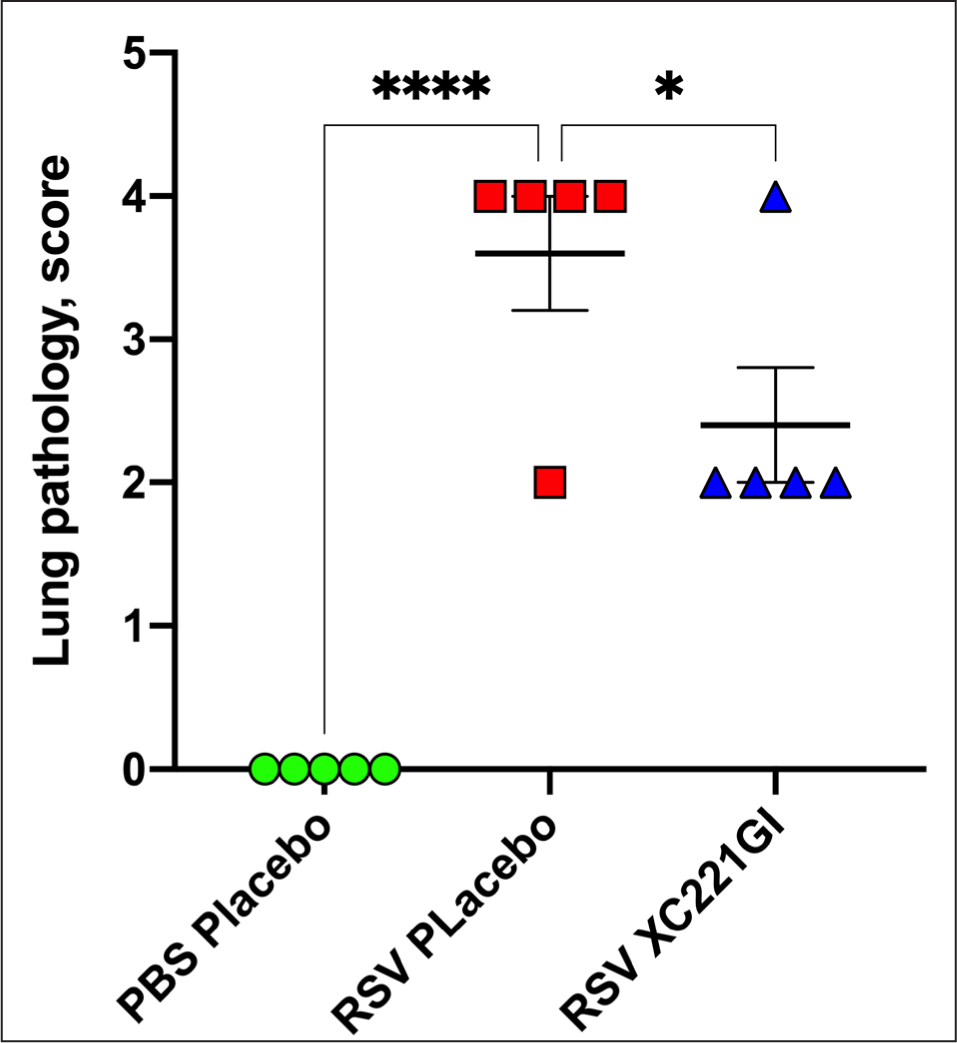
The administration of XC221GI to animals resulted in a significant decrease in the severity of alveolitis and interstitial pneumonia in the lungs compared to the control group of animals infected with RSV and treated with water without XC221GI (RSV Placebo) (Fig. 2).
The effect of XC221GI (20 mg/kg/day) on the viral load and RSV-induced lung pathology in cotton rats was also studied in the immunosuppressed animals. We showed that treatment with XC221GI leads to a significant decrease in RSV titer in the lungs of immunosuppressed cotton rats compared to the animals in the control group (Fig. 3).

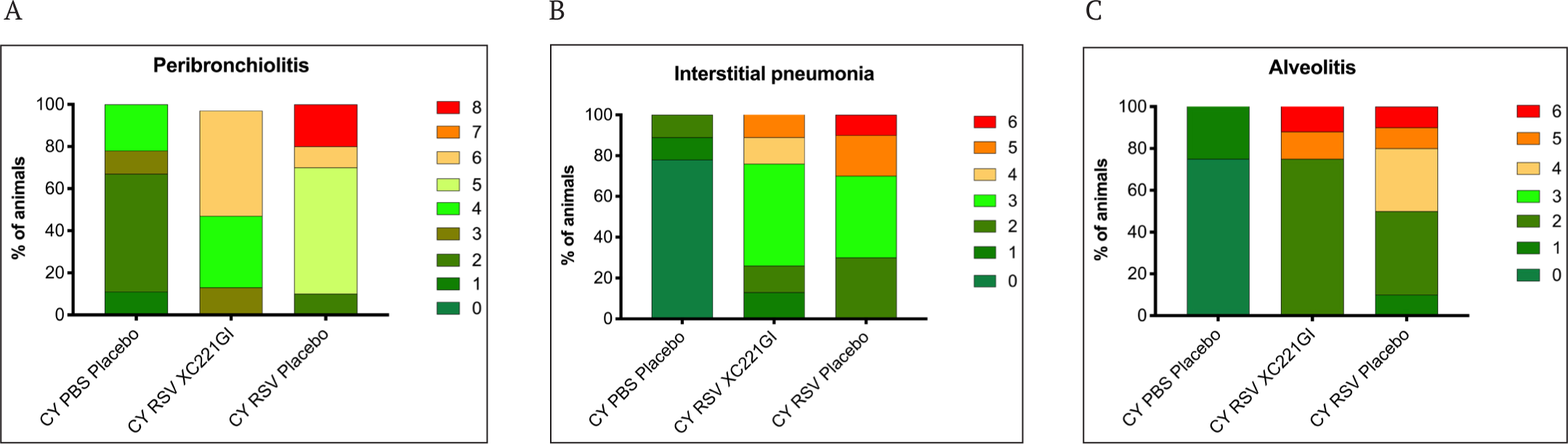
The maximum pathology in the lungs of immunosuppressed animals was observed on the 10th day after infection. The detailed study of pathology in the lungs of cotton rats included analysis of the tissue specimens stained with H&E, Duffy, PAS, and toluidine blue for the presence of: inflammatory cellular infiltrates in the peribronchiolar connective tissue and, to a lesser extent, in the bronchiolar epithelium (peribronchiolitis); inflammation in the alveolar septal interstitium near and inside the terminal bronchoalveolar canals (interstitial pneumonia) as well as the exudates of inflammatory cells in the alveolar air space (alveolitis). The pathology score was assessed and summed up for each type of analysis.
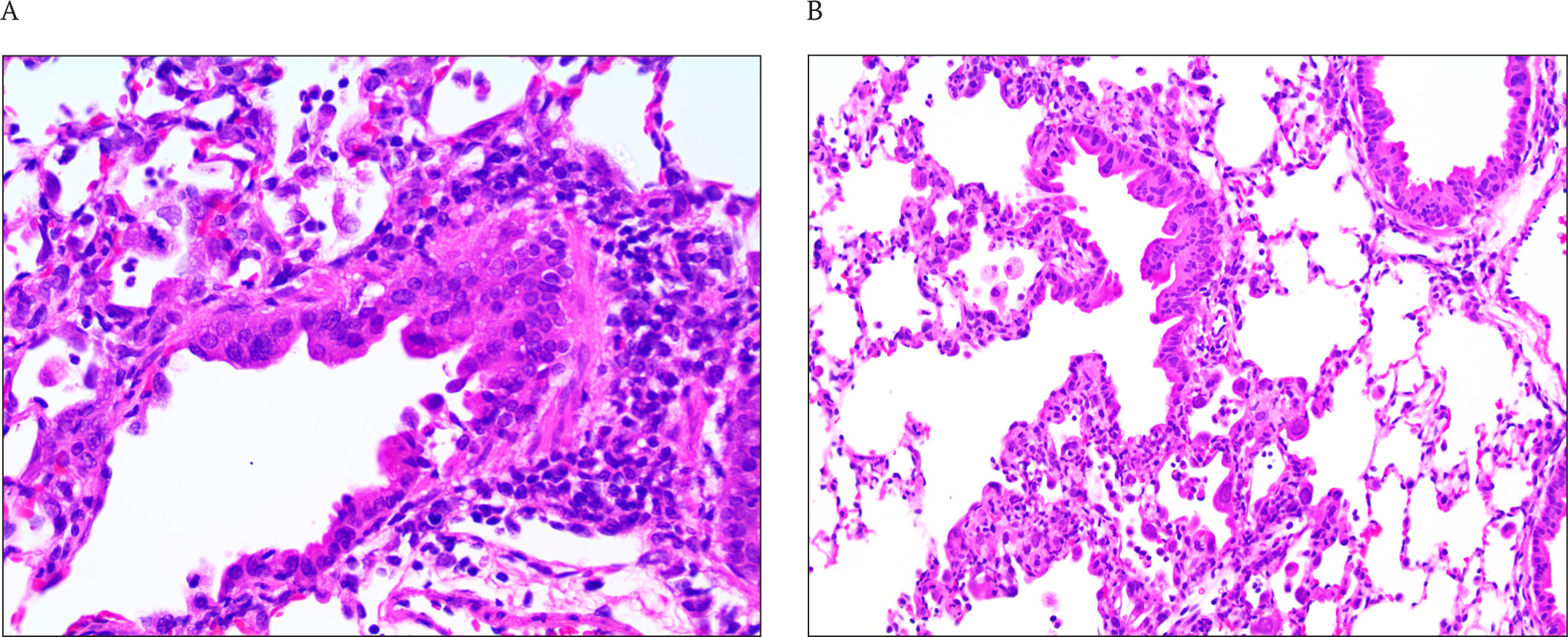
RSV infection caused peribronchiolitis, which is characterized by the presence of submucosal peribronchiolar interstitial infiltrates of predominantly mononuclear inflammatory cells with an admixture of eosinophils and neutrophils. The staining of lung sections with H&E revealed peribronchiolitis in all the groups of RSV-infected animals. The pathology was the most pronounced in the animals of the control group receiving a placebo. In the more affected specimens, the peribronchiolitis extended deeply, involving the terminal bronchioles. Eosinophils were a minor component of the inflammatory process. There were no animals with severe pathology in the group of cotton rats treated with XC221GI (Fig. 4, 5 A). The evaluation of the severity of interstitial inflammatory cell infiltrates also showed a decrease in the severity of interstitial pneumonia in XC221GI-treated cotton rats compared to the animals in the placebo group (Fig. 5 B).
Effect of XC221GI on neutrophil recruitment and the production of chemokines CXCL10, CXCL9, and CXCL11 in the lungs of mice
An increase in inflammation followed by the development of MIS is a frequent complication in humans infected with the SARS-CoV-1 and SARS-CoV-2 viruses [42, 43]. The CXCL10-CXCR3 axis chemokine signaling was shown to be a critical factor in the development of pulmonary pathology in COVID-19 patients [44]. The increased expression of CXCL10 and related ligands CXCL9 and CXCL11 of the CXCR3 receptor has been found in BAL and peripheral blood mononuclear cells (PBMC) of patients with severe COVID-19. A population of neutrophils expressing the CXCR3 receptor was revealed in infected lungs [45]. Since IFNγ signaling through the airway epithelium induces chemokines, we developed a model for neutrophil influx and the induction of chemokines CXCL10, CXCL9, and CXCL11 in mouse lungs after the i.n. administration of IFNγ to animals. We showed that a significant increase in the level of neutrophils in bronchoalveolar lavage is observed after a single injection of IFNγ compared to intact animals already after 4 h and continues to increase up to 12 h (Fig. 6 A). In addition, the inoculation of IFNγ in 2 h led to a statistically significant increase in the level of CXCL10 in the BAL that persisted within 12 h (Fig. 6 B).
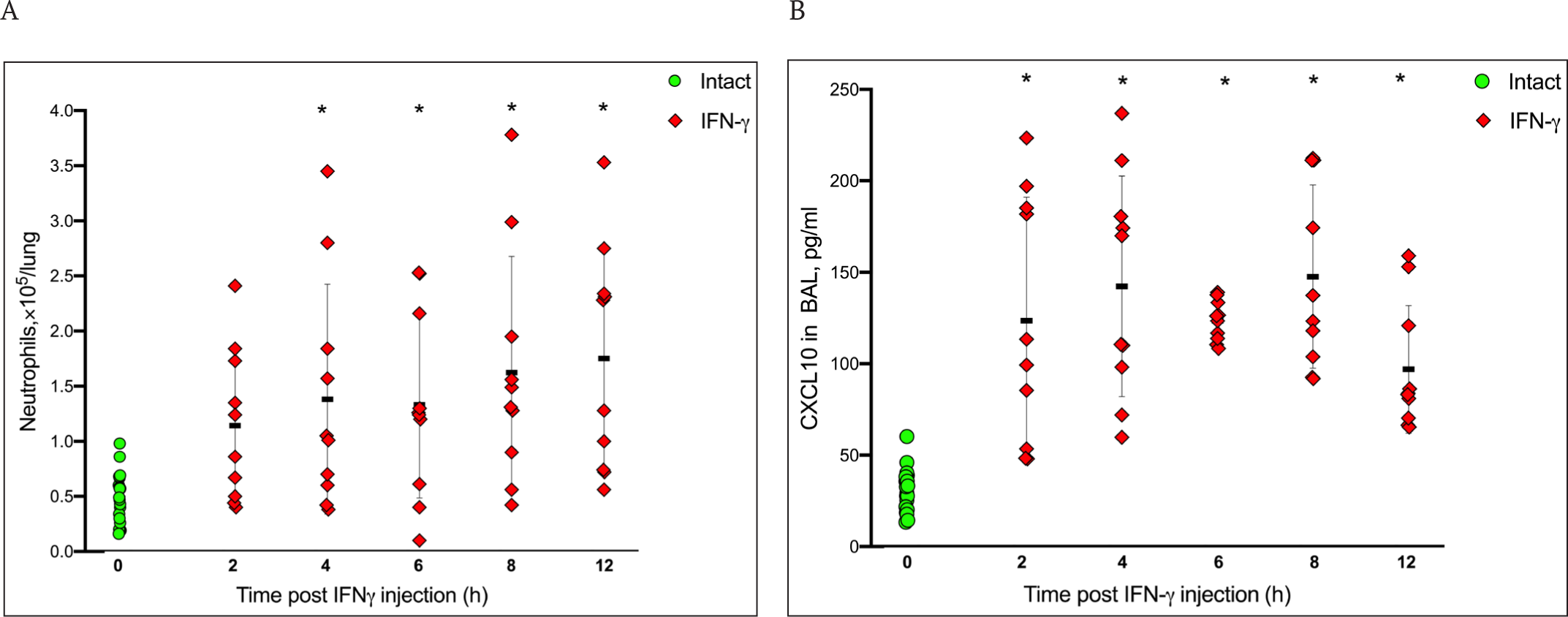
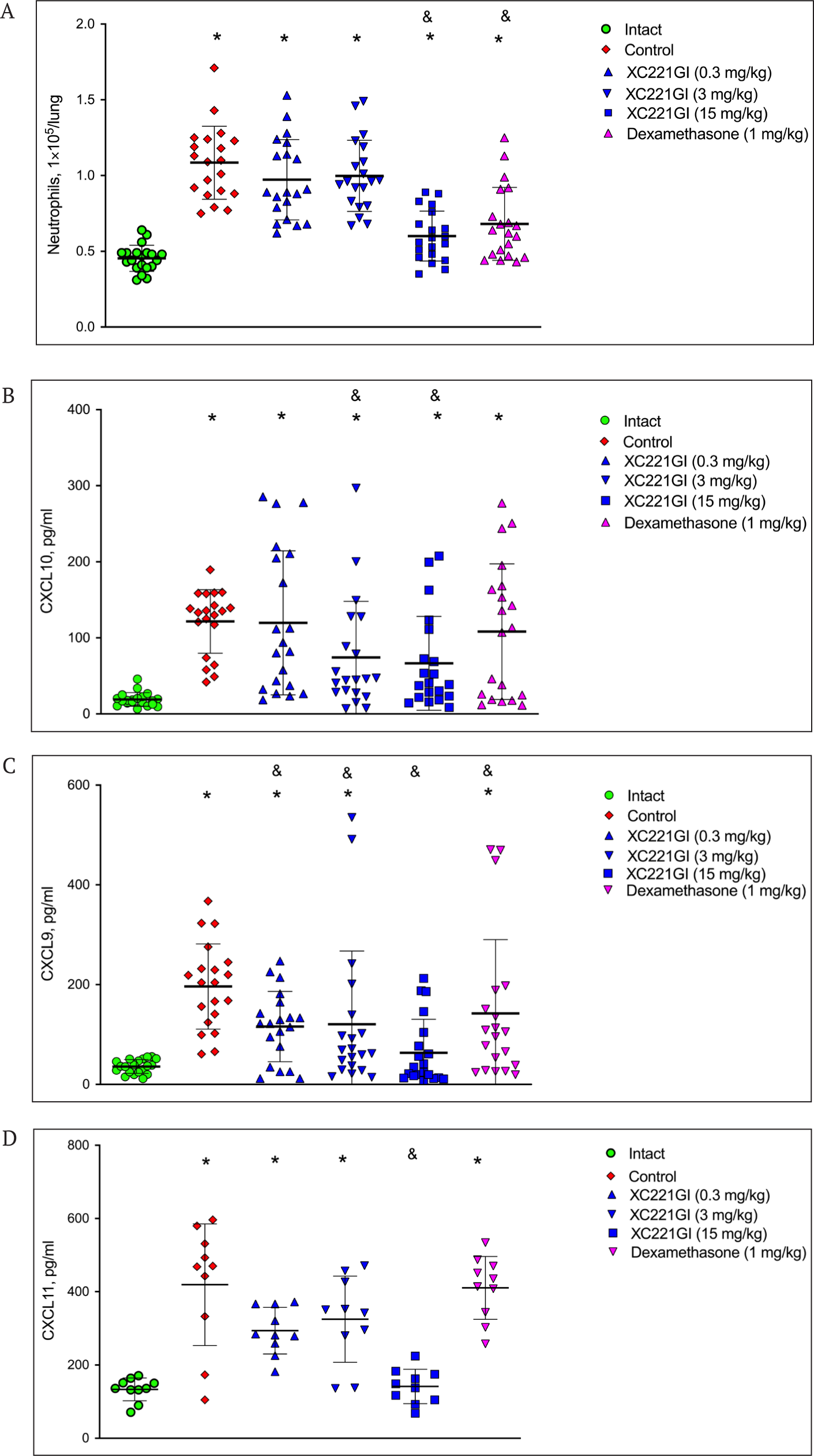
Next, we studied the effect of the administration of XC221GI to mice on the level of neutrophils and chemokines CXCL10, CXCL9, and CXCL11 in the lungs after a single injection of IFNγ. We have shown that the treatment of mice with XC221GI at a dose of 15 mg/kg in 8-12 h after the injection of IFNγ resulted in a statistically significant decrease in the number of neutrophils in BAL compared with the control animals treated with a placebo (Fig. 7 A). The effect of XC221GI was comparable to that of dexamethasone (1 mg/kg). The study of the effect of XC221GI on chemokines – ligands of the CXCR3 receptor – showed that a single administration of XC221GI (at doses of 3 and 15 mg/kg) significantly reduced the level of induction of CXCL10 and CXCL9 at 8-12 h after the IFNγ injection (Fig. 7 B, C). The administration of dexamethasone led to a significant decrease in CXCL9 levels. The drug XC221GI (at a dose of 15 mg/kg), but not dexamethasone, led to a significant decrease in CXCL11 in bronchoalveolar lavage at 8 h after the IFNγ injection (Fig. 7 D).
DISCUSSION
IL6 is one of the main pro-inflammatory cytokines produced in the course of infection with respiratory viruses [46, 47]. Blocking of the IL6-R receptor with the monoclonal antibody tocilizumab was shown to lead to rapid improvement in patients with severe COVID-19 [48]. Therefore, IL6 may serve as a relevant therapeutic target for the treatment of virus-induced inflammation. Since an elevated level of IL8 is observed in the blood plasma of patients with a severe course of COVID-19 disease, this pro-inflammatory cytokine can also serve as a target for the treatment of virus-induced inflammation [49]. IL8 is involved in a massive influx of neutrophils into the interstitium, perivascular, and peribronchial spaces of the lungs leading to their damage [50, 51]. In addition, IL8 plays a key role in the development of the prothrombotic neutrophil phenotype in patients. Blocking the IL8 signaling with an anti-IL8 monoclonal antibody or a CXCR1/2 receptor blocker (reparixin) [52] was shown to decrease neutrophil activation in vitro and to reduce the severity of acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 in mice [49].
We studied the effect of XC221GI on the viral inflammation in experiments with RSV infection in vitro and in vivo and showed that this drug effectively suppresses the RSV-induced production of IL6 (45-85%) and IL8 (40%) in human epithelial carcinoma cells. Importantly, XC221GI did not affect the spontaneous production of IL6 and IL8 cytokines in the absence of RSV virus.
Respiratory viruses enter the epithelial cells of the upper respiratory tract through the bronchiolar epithelium and then penetrate the lower respiratory tract causing bronchiolitis. RSV is responsible for 70% of all bronchiolitis in children and is the main cause of death in children up to two years old [53]. Rhinoviruses cause bronchiolitis in children less frequently than RSV. Seasonal coronaviruses did not cause bronchiolitis, but the situation has changed with the SASR-CoV-2 variant B.1.1.529 (Omicron). The Omicron strain multiplies less effectively in the lungs of animals compared to the previous variants of SARS-CoV-2 [54] and less often leads to pneumonia, but causes a sharp morbidity increase among children with a high incidence of severe cases, including croup, bronchiolitis, and bronchial obstruction [55].
RSV-induced bronchiolitis is characterized by neutrophilia, the release of inflammatory cytokines, and the hypersecretion of mucus that can occlude the lumen of the bronchioles, contributing to bronchiolar obstruction [56]. An RSV infection in cotton rats with the development of interstitial pneumonia, alveolitis, and proliferative bronchiolitis by the 5th-6th day after the infection is the most appropriate animal model [57]. Using this model, we studied the effect of XC221GI on the virus-induced lung pathology of these animals. We have shown that the administration of XC221GI for 5 days resulted in a significant reduction in the severity of interstitial pneumonia and alveolitis in animals.
The infection of immunosuppressed cotton rats by the RSV virus caused a typical bronchiolar and interstitial inflammatory response in the lungs with the formation of peribronchiolar and alveolar infiltrates of mononuclear inflammatory cells as well as the induction of neutrophils and eosinophils. The treatment of animals with XC221GI for 9 days led to the statistically significant decrease in the viral load in the lungs and a decrease in pathology revealed by the analysis of peribronchiolar, interstitial, and alveolar changes. The cases of peribronchiolitis and interstitial pneumonia with severe damage to the lung tissue were not detected in XC221GI treated animals in contrast to the animals of the control group.
The elevated levels of chemokines CXCL10, CXCL9, CXCL11 – ligands of the CXCR3 receptor – are also observed in the severe cases of the virus-induced inflammation of the respiratory tract in humans. CXCR3 is considered an inflammatory chemokine receptor because it is expressed by leukocytes that migrate to the sites of inflammation. Elevated levels of CXCL10 in the lungs cause infiltration by neutrophils expressing the CXCR3 receptor. Such neutrophils are found in patients with chronic lung disease but not in healthy people [58]. The CXCL10-CXCR3 signaling is a critical factor of the lung pathology enhancement in multisystem inflammation due to its effect on the chemotaxis of neutrophils [44]. It is also associated with Th1-type diseases, including autoimmune, cardiovascular, and infectious diseases [59, 60].
Being a regulator of the immune response to infection, IFNγ induces all three CXCR3 ligands – CXCL10, CXCL9, and CXCL11 – by activating the JAK/STAT/Akt signaling pathway [61-63]. The chemokines CXCL10, CXCL9, and CXCL11 have a similar protein sequence composition with up to 40% identity [64]. The chemokines CXCL9 and CXCL10 induce the polarization of Th1/Th17 effector cells and support the inflammatory response [65]. CXCL10 is involved in chemotaxis, apoptosis and angiostasis, and it is the main marker of ARDS [26, 66]. In severe COVID-19 cases, the chemokines CXCL10, CXCL9, and CXCL11 are abundantly induced in human lung epithelial cells. A significant increase in the level of CXCL9, which is included in the list of 10 chemokines associated with an increased risk of mortality [6], was noticed in the serum of COVID-19 patients with generalized inflammation [31]. The determination of the CXCL9, CXCL10, and CXCL11 chemokines levels allows to identify the COVID-19 patients with an increased risk of severe complications. Therefore, by the regulation of the chemokine levels, it is possible to prevent the severe complications in patients infected with SARS-CoV-2.
The CXCL10-CXCR3 axis is an important therapeutic target in the treatment of the acute phase of pulmonary inflammation. We studied the effect of XC221GI on the influx of neutrophils into the bronchoalveolar space of the lungs of mice and the induction of chemokines CXCL10, CXCL9, and CXCL11 after the i.n. administration of IFNγ to animals. Using the model that we developed for these experiments, we showed that a single administration of XC221GI leads to a significant decrease in the influx of neutrophils into the lungs and a decrease in the production of all three ligands – CXCL9, CXCL10, and CXCL11 – as early as 8-12 h after the administration of IFNγ. The effect of XC221GI on the pulmonary influx of neutrophils and level of CXCL9 was comparable to that of dexamethasone. No statistically significant effect of dexamethasone on CXCL10 and CXCL11 levels was noted in this model.
The obtained results of a preclinical study of the XC221GI revealed the unique anti-inflammatory mode of action of this compound – the ability to control the level of production of the key inflammatory and hyperinflammatory markers in viral infection. The proven control of IL6 and IL8 production as well as the levels of CXCL10, CXCL9, and CXCL11 by XC221GI is a key property of this compound that justifies its use against the RSV infection and possibly against the SARS-CoV-2.
These results suggest that XC221GI can be used as a medicine for the proactive anti-inflammatory therapy in RSV to reduce viral damage to the lung tissue and in COVID-19 cases at all stages of the inflammatory process to prevent the development of hypercytokinemia and its complications, including thromboembolic disorders and the dysfunction of the key vital organs.

