- Record: found
- Abstract: found
- Article: found
Development and Validation of a New Score to Assess the Risk of Posttransplantation Diabetes Mellitus in Kidney Transplant Recipients

Read this article at
Abstract
Background.
Posttransplantation diabetes mellitus (PTDM) is a serious complication of solid organ transplantation. It is associated with major adverse cardiovascular events, which are a leading cause of morbidity and mortality in transplant patients. This study aimed to develop and validate a score to predict the risk of PTDM in kidney transplant recipients.
Methods.
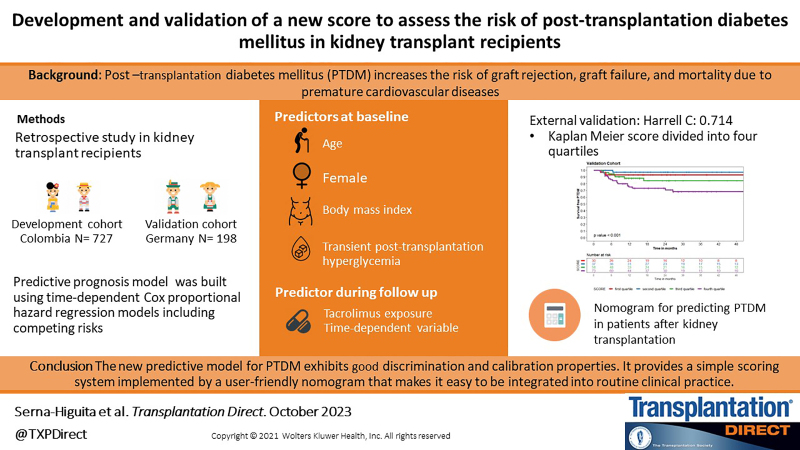
A single-center retrospective cohort study was conducted in a tertiary care hospital in Medellín, Colombia, between 2005 and 2019. Data from 727 kidney transplant recipients were used to develop a risk prediction model. Significant predictors with competing risks were identified using time-dependent Cox proportional hazard regression models. To build the prediction model, the score for each variable was weighted using calculated regression coefficients. External validation was performed using independent data, including 198 kidney transplant recipients from Tübingen, Germany.
Results.
Among the 727 kidney transplant recipients, 122 developed PTDM. The predictive model was based on 5 predictors (age, gender, body mass index, tacrolimus therapy, and transient posttransplantation hyperglycemia) and exhibited good predictive performance (C-index: 0.7 [95% confidence interval, 0.65-0.76]). The risk score, which included 33 patients with PTDM, was used as a validation data set. The results showed good discrimination (C-index: 0.72 [95% confidence interval, 0.62-0.84]). The Brier score and calibration plot demonstrated an acceptable fit capability in external validation.
Abstract
Related collections
Most cited references51
- Record: found
- Abstract: not found
- Article: not found
A Proportional Hazards Model for the Subdistribution of a Competing Risk
- Record: found
- Abstract: found
- Article: not found
Assessing the performance of prediction models: a framework for traditional and novel measures.
- Record: found
- Abstract: not found
- Article: not found