- Record: found
- Abstract: found
- Article: found
Insights into N6-methyladenosine and programmed cell death in cancer
Read this article at
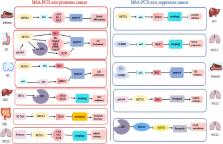
Abstract
N6-methyladenosine (m6A) methylation, the most common form of internal RNA modification in eukaryotes, has gained increasing attention and become a hot research topic in recent years. M6A plays multifunctional roles in normal and abnormal biological processes, and its role may vary greatly depending on the position of the m6A motif. Programmed cell death (PCD) includes apoptosis, autophagy, pyroptosis, necroptosis and ferroptosis, most of which involve the breakdown of the plasma membrane. Based on the implications of m6A methylation on PCD, the regulators and functional roles of m6A methylation were comprehensively studied and reported. In this review, we focus on the high-complexity links between m6A and different types of PCD pathways, which are then closely associated with the initiation, progression and resistance of cancer. Herein, clarifying the relationship between m6A and PCD is of great significance to provide novel strategies for cancer treatment, and has a great potential prospect of clinical application.
Related collections
Most cited references141
- Record: found
- Abstract: found
- Article: not found
Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq.
- Record: found
- Abstract: found
- Article: not found
N6-Methyladenosine in Nuclear RNA is a Major Substrate of the Obesity-Associated FTO
- Record: found
- Abstract: found
- Article: not found