- Record: found
- Abstract: found
- Article: found
Sulforaphane exposure impairs contractility and mitochondrial function in three-dimensional engineered heart tissue

Read this article at
Abstract
Sulforaphane (SFN) is a phytochemical compound extracted from cruciferous plants, like broccoli or cauliflower. Its isothiocyanate group renders SFN reactive, thus allowing post-translational modification of cellular proteins to regulate their function with the potential for biological and therapeutic actions. SFN and stabilized variants recently received regulatory approval for clinical studies in humans for the treatment of neurological disorders and cancer. Potential unwanted side effects of SFN on heart function have not been investigated yet. The present study characterizes the impact of SFN on cardiomyocyte contractile function in cardiac preparations from neonatal rat, adult mouse and human induced-pluripotent stem cell-derived cardiomyocytes. This revealed a SFN-mediated negative inotropic effect, when administered either acutely or chronically, with an impairment of the Frank-Starling response to stretch activation. A direct effect of SFN on myofilament function was excluded in chemically permeabilized mouse trabeculae. However, SFN pretreatment increased lactate formation and enhanced the mitochondrial production of reactive oxygen species accompanied by a significant reduction in the mitochondrial membrane potential. Transmission electron microscopy revealed disturbed sarcomeric organization and inflated mitochondria with whorled membrane shape in response to SFN exposure. Interestingly, administration of the alternative energy source l-glutamine to the medium that bypasses the uptake route of pyruvate into the mitochondrial tricarboxylic acid cycle improved force development in SFN-treated EHTs, suggesting indeed mitochondrial dysfunction as a contributor of SFN-mediated contractile dysfunction. Taken together, the data from the present study suggest that SFN might impact negatively on cardiac contractility in patients with cardiovascular co-morbidities undergoing SFN supplementation therapy. Therefore, cardiac function should be monitored regularly to avoid the onset of cardiotoxic side effects.
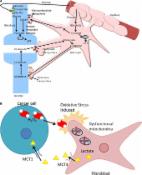
Graphical abstract
Summary scheme of the functional effects of SFN exerted in cardiomyocytes: decline in force, elevation of diastolic tension and alteration of mitochondrial function and metabolism. MM: mitochondrial membrane potential; MPC: mitochondrial pyruvate carrier; mito: mitochondria; PC: pyruvate carboxylase; PDH: pyruvate dehydrogenase; Mitochondrium adapted from Servier Medical ART: SMART ( smart.servier.com).
Highlights
-
•
Sulforaphane has negative inotropic effects and increases diastolic tension.
-
•
Sulforaphane exposure increases lactate levels and mitochondrial ROS production and reduces mitochondrial membrane potential.
-
•
l-glutamine supplementation rescues the sulforaphane-mediated reduction in force development.
-
•
Sulforaphane plasma levels and cardiac function should be monitored to avoid unwanted cardiac side effects in patients.
Related collections
Most cited references39
- Record: found
- Abstract: found
- Article: not found
The Warburg Effect: How Does it Benefit Cancer Cells?
- Record: found
- Abstract: found
- Article: not found
Energy metabolic phenotype of the cardiomyocyte during development, differentiation, and postnatal maturation.
- Record: found
- Abstract: found
- Article: found
