- Record: found
- Abstract: found
- Article: found
Magnolol Ameliorates Ligature-Induced Periodontitis in Rats and Osteoclastogenesis: In Vivo and In Vitro Study
Read this article at
Abstract
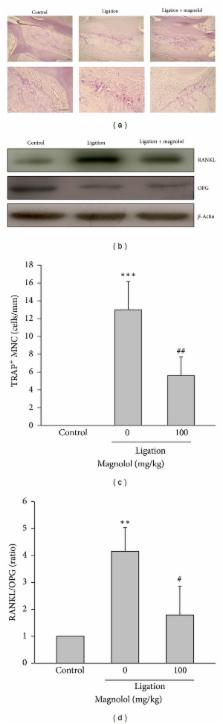
Periodontal disease characterized by alveolar bone resorption and bacterial pathogen-evoked inflammatory response has been believed to have an important impact on human oral health. The aim of this study was to evaluate whether magnolol, a main constituent of Magnolia officinalis, could inhibit the pathological features in ligature-induced periodontitis in rats and osteoclastogenesis. The sterile, 3–0 (diameter; 0.2 mm) black braided silk thread, was placed around the cervix of the upper second molars bilaterally and knotted medially to induce periodontitis. The morphological changes around the ligated molars and alveolar bone were examined by micro-CT. The distances between the amelocemental junction and the alveolar crest of the upper second molars bilaterally were measured to evaluate the alveolar bone loss. Administration of magnolol (100 mg/kg, p.o.) significantly inhibited alveolar bone resorption, the number of osteoclasts on bony surface, and protein expression of receptor activator of nuclear factor- κ B ligand (RANKL), a key mediator promoting osteoclast differentiation, in ligated rats. Moreover, the ligature-induced neutrophil infiltration, expression of inducible nitric oxide synthase, cyclooxygenase-2, matrix metalloproteinase (MMP)-1 and MMP-9, superoxide formation, and nuclear factor- κ B activation in inflamed gingival tissues were all attenuated by magnolol. In the in vitro study, magnolol also inhibited the growth of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans that are key pathogens initiating periodontal disease. Furthermore, magnolol dose dependently reduced RANKL-induced osteoclast differentiation from RAW264.7 macrophages, tartrate-resistant acid phosphatase (TRAP) activity of differentiated cells accompanied by a significant attenuation of resorption pit area caused by osteoclasts. Collectively, we demonstrated for the first time that magnolol significantly ameliorates the alveolar bone loss in ligature-induced experimental periodontitis by suppressing periodontopathic microorganism accumulation, NF- κ B-mediated inflammatory mediator synthesis, RANKL formation, and osteoclastogenesis. These activities support that magnolol is a potential agent to treat periodontal disease.
Related collections
Most cited references37
- Record: found
- Abstract: found
- Article: not found
Inflammation and uncoupling as mechanisms of periodontal bone loss.
- Record: found
- Abstract: found
- Article: not found
Surface RANKL of Toll-like receptor 4-stimulated human neutrophils activates osteoclastic bone resorption.
- Record: found
- Abstract: found
- Article: not found