- Record: found
- Abstract: found
- Article: found
Phasic firing of dopaminergic neurons in the ventral tegmental area triggers peripheral immune responses
Read this article at
Abstract
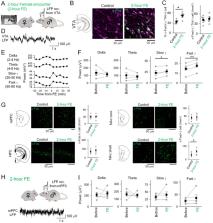
Dopaminergic neurons in the ventral tegmental area (VTA) play a crucial role in the processing of reward-related information. Recent studies with pharmacological manipulations of VTA neuronal activity demonstrated a VTA-induced immunoenhancement in peripheral organs. Here, to examine the detailed physiological dynamics, we took an optogenetic approach in which VTA dopaminergic neurons were selectively activated with millisecond precision. Optogenetic phasic, rather than tonic, stimulation of VTA dopaminergic neurons increased serum cytokine levels, such as IL-2, IL-4 and TNF-α. These results provide direct evidence to link dopaminergic neuronal phasic firing to peripheral immunity. Next, we tested whether cytokine induction in male mice was boosted by female encounters, a natural condition that induces increased active VTA neurons and gamma power. Female encounters increased serum IL-2 levels, which were abolished by pharmacological inhibition of VTA neuronal activity. Taken together, our results highlight the importance of the brain reward system in the treatment and management of immune-related disorders.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning.
- Record: found
- Abstract: found
- Article: not found
Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons
- Record: found
- Abstract: found
- Article: not found